NCATS Team’s Rapid Test Finds Promising Therapies for Myositis
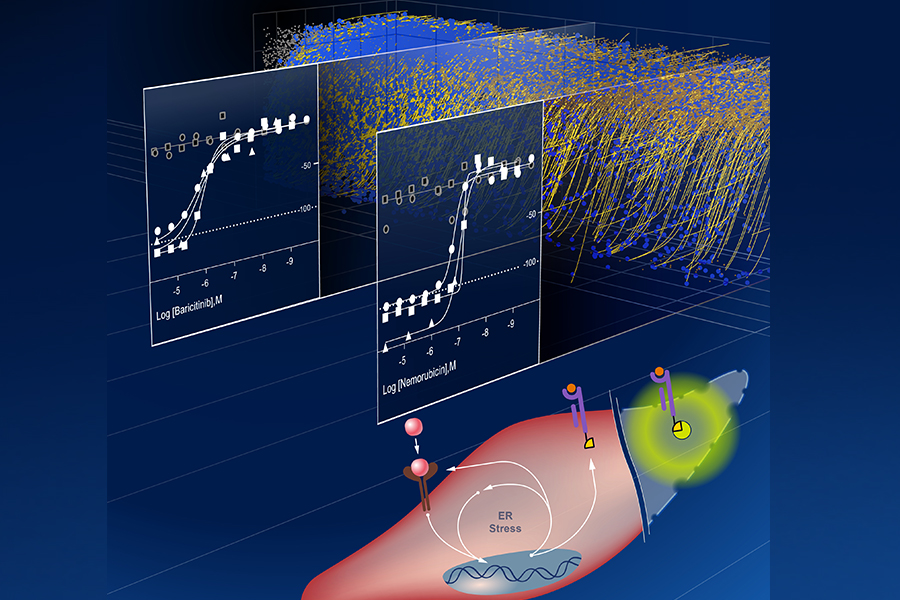
NCATS scientists have developed an approach to rapidly test thousands of existing drugs to identify promising therapies for myositis, a rare autoimmune disease. Results from hundreds of drug tests on human muscle cells (in background) reveal two potential candidates (in foreground) to treat the disease. (NCATS and NHGRI; created by Darryl Leja, NHGRI)
October 22, 2020
Idiopathic inflammatory myopathy, also known as myositis, is a rare muscle disease with no effective therapies and few experimental treatments in development. But a new research initiative by NCATS scientists could point to promising new options. They swiftly tested thousands of existing drugs to find therapeutics that may target a key trigger in myositis.
Myositis is an autoimmune disease characterized by chronic inflammation of the muscles. A person’s own immune system attacks their muscle cells, leading to skeletal muscle weakness and pain, as well as complications in the skin, lungs and circulatory system. Myositis affects an estimated 2,000 to 4,000 people in the United States, and incidence of the disease is on the rise.
“There is no specifically approved therapy for myositis, and there is no cure,” explained Travis B. Kinder, Ph.D., a postdoctoral research fellow at NCATS and the study’s corresponding author. “Our goal was to find new therapeutics for this rare condition.”
Current treatments include anti-inflammatory steroids, as well as drugs that suppress the immune system and biologic agents that modulate the immune system. However, first-line options, such as high-dose prednisone, can have serious side effects — particularly for children with myositis — and current therapies do not stop muscle inflammation completely or return muscles to normal.
With funding from the Cure Juvenile Myositis Foundation, Kinder and his fellow study investigators, NCATS senior research scientist Patricia K. Dranchak, Ph.D., and NCATS principal investigator James Inglese, Ph.D., developed an approach to rapidly test thousands of existing drugs and identify candidates for further study. Their findings appeared online May 27 in ACS Chemical Biology.
The first step was to identify a promising therapeutic target. The NCATS researchers picked a target that may contribute to the immune system’s attack on muscle cells: the inflammatory pathway between type I interferon (IFN) and major histocompatibility complex (MHC) class I. IFN modulates the immune system’s response to infection, and MHC class I attracts the immune system to attack pathogens and diseased cells.
In the weakened skeletal muscles of myositis, growing evidence links high levels of type I IFN and the overproduction of MHC class I. That duo typically springs into action when a virus attacks. However, scientists have yet to find evidence of a viral trigger in myositis, which means IFN and production of MHC class I could be therapeutic targets in myositis.
Using CRISPR/Cas9 genome editing, the research team developed a line of human muscle cells to test the IFN–MHC class I pathway. They then began the hunt for drugs to stop the cycle of inflammation and autoimmunity in myositis, turning to NCATS’ library of thousands of investigational and U.S. Food and Drug Administration (FDA)-approved drug compounds.
The researchers analyzed the compounds in that library through a process known as quantitative high-throughput screening (qHTS), which can test thousands of compounds and drugs at the same time. Using qHTS, they rapidly tested 4,679 unique compounds at assorted concentrations to assess their pharmacological effects on the targeted inflammatory pathway. Of the thousands of drugs tested, NCATS scientists focused on 12 drugs and compounds that showed the most promise.
The most effective drug proved to be the antibiotic echinomycin, which had been developed and abandoned as a potential cancer therapy. Drugs that inhibit cellular signaling proteins called kinases were the largest class of promising compounds screened. Three FDA-approved Janus kinase inhibitors were nearly 100% effective at inhibiting the targeted pathway: ruxolitinib, baricitinib and tofacitinib. Of the three, ruxolitinib was most potent.
Several compounds that interfere with gene activity also actively blocked the targeted pathway. Those compounds include FDA-approved panobinostat, vorinostat and doxorubicin. Another promising investigational drug, givinostat, is already in trials for Duchenne muscular dystrophy, a rare disease that also features muscle inflammation.
The Duchenne therapeutic connection highlights how drugs that target the type I IFN–MHC class I pathway could have an impact beyond myositis, Kinder explained. Other autoimmune diseases that might benefit from such treatments include rheumatoid arthritis, Sjögren’s syndrome, systemic lupus erythematosus and systemic sclerosis.
One of the next research steps would be animal-model myositis trials, particularly for some of the less-studied drugs, Kinder added. He and his colleagues plan to continue screening new Janus kinase inhibitors and novel compounds, explore potential environmental triggers for myositis, and search the genome to discover new target genes and pathways for novel therapeutic interventions.


