New NCATS-Supported Test Could Provide Faster Diagnosis of Deadly Disease
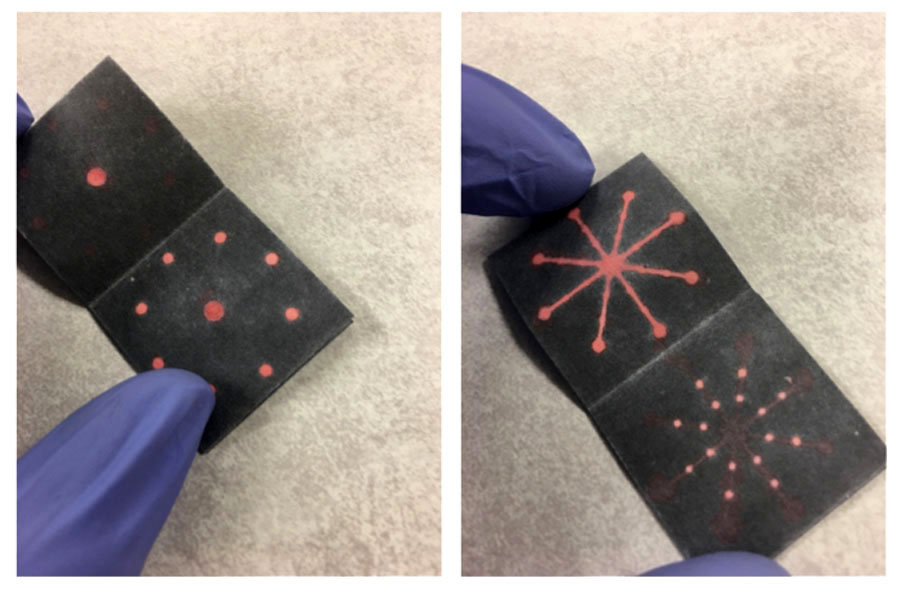
Method of detecting nucleic acids using three-dimensional paper microfluidic devices. A sample is loaded on the one dot on the left. It is dispersed into seven different locations for the multiplexed detection of seven pathogens and one location for the control. (GoDx Photo)
Every year, diarrheal disease kills more than 1 million people, mostly children in the developing world. To treat the illness effectively, health care providers need to know its cause. Currently, a diagnosis requires sending a stool sample to a lab for expensive tests, then waiting to find out if the patient has a bacterial or viral infection, a parasite, or some other problem. By the time the results come back, the patient has missed days of potentially lifesaving treatment.
To address this challenge, Chang Hee Kim, Ph.D., co-founded the biotechnology company GoDx with a mission to develop a simple paper test for diarrheal disease. The test does not need electricity and can detect seven common causes of diarrhea in less than 30 minutes.
GoDx has a clinical cooperative research and development agreement to develop the product with Wendy A. Henderson, Ph.D., M.S.N., C.R.N.P., a digestive diseases researcher at NIH’s National Institute of Nursing Research (NINR). Data generated through that collaboration made it possible for Kim to apply for and receive a Phase 1 Small Business Innovation Research (SBIR) grant from NCATS in 2017. NCATS chose to fund the application because it supported the development of a platform technology that could be used to diagnose many different diseases. GoDx plans to apply the technology to urinary tract infections, sepsis and sexually transmitted infections, which all involve the rapid, multiplexed detection of pathogens at the point of care or point of need. The technology will enable syndromic testing in a pocket-sized package. SBIR Phase 1 funding supports small businesses in the early stages, when the companies are trying to make sure that their proposed research and development efforts will work and be worthwhile.
“It’s so valuable that NCATS is willing to fund research and development for this early-stage technology,” Kim said. “At the beginning of technology development, it is very hard to find investors.”

GoDx co-founder Chang Hee Kim, Ph.D., and NIH researcher Wendy A. Henderson, Ph.D., M.S.N., C.R.N.P., co-developed a new test for diarrheal disease.
The Phase 1 SBIR funding made Kim eligible to take part in the I-Corps™ at NIH program, which provides mentoring and training for entrepreneurs. As part of I-Corps, Kim and his colleagues interviewed 130 patients, doctors and others about the test and received input about improving and distributing it.
In July 2018, GoDx received Phase 2 SBIR funding from NCATS to continue test development. The next step is a study with NINR to try out the test on volunteers; anyone with diarrhea near Bethesda, Maryland, can sign up. At the same time, study participants’ stool samples will be tested via more traditional laboratory procedures. Comparing the two results will indicate whether the new test is accurate.
“This is just one example of how NCATS’ SBIR funding can support translational research advances that have commercial as well as lifesaving potential,” said Lili Portilla, M.P.A., director of NCATS’ SBIR and Small Business Technology Transfer programs.
Read more from The New Gastroenterologist.
Posted August 2018


