NCATS Strategic Plan
Our mission is to turn research observations into health solutions through translational science. We define translational science as the field that generates scientific and operational innovations to overcome long-standing challenges along the translational research pipeline.
2024–2029 Planning
Contact
Overview
The NCATS Strategic Plan for 2024–2029 will guide the center in working toward the vision of Director Joni L. Rutter, Ph.D., of more treatments for all people more quickly and toward achieving our audacious goals in meaningful and achievable ways to move the field of translational science forward. Read more about the strategic plan in the April Director’s Message.
The strategic plan will create a roadmap for the next several years (2024–2029) and will enable planning for longer-term efforts that may be started within this time frame. The strategic plan will support our mission, echo our values, and be bold yet attainable.
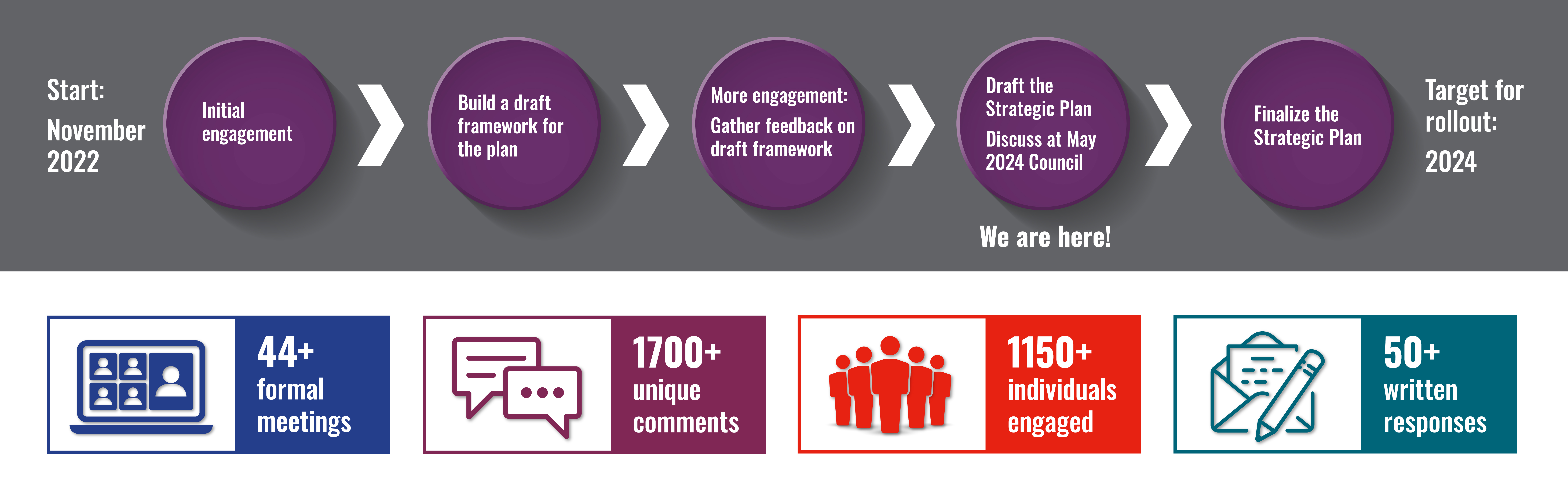
Timeline Overview
Engagement Activities
Thank you to everyone who submitted responses to our recent request for information (RFI), which closed on November 1, 2023. We appreciate you sharing your feedback and insight on the NCATS draft strategic plan framework as we begin drafting the NCATS strategic plan. Your engagement is an important part of our process for developing the NCATS strategic plan and further shaping NCATS’ goals and objectives.
To inform the draft strategic plan framework, NCATS hosted two virtual strategic planning roundtable discussions on May 9 and 10, 2023. Dr. Rutter gave an overview of her vision for NCATS and provided information on the strategic plan; participants then split into four breakout groups to offer their perspectives. Discussions were based on four translational science areas: Research, Training and Education, Patient and Community Engagement, and DEIA. View the presentation slides and meeting summary.
NCATS has also completed the following engagement activities:
- Two public roundtables
- Communities supporting research for the Clinical and Translational Science Awards (CTSA) Program
- Communities supporting research for the Rare Disease Clinical Research Network (RDCRN)
- Public Request for Information (RFI)
- Email input
- All NCATS Divisions, Offices and leadership groups
NCATS FY 2024–2029 Draft Strategic Plan Framework
This framework builds on the input received through several engagement meetings that NCATS leadership had with both the public and with NCATS personnel and was shared broadly in a request for information (RFI), which closed on November 1, 2023. The framework and input received by NCATS will be used in the development of the draft NCATS Strategic Plan.
Goal 1: Apply Approaches to Foster the Identification of, Development of, and Access to More Treatments: NCATS applies strategies, techniques, and technologies to address unmet needs. We look for commonalities across diseases and develop platforms with multiple uses. Bringing more treatments also means applying translational science to identify and address the potential for disease, across demographics, as well as identifying ways to ensure interventions are available to those that need them.
Goal 1 includes, but is not limited to, the following themes:
- Developing treatment strategies for addressing multiple diseases at a time through targeted approaches
- Addressing development of treatments for rare diseases and other unmet needs
- Developing a clinical trial ecosystem ready to address long-standing and urgent public health priorities
- Supporting early indication of and prevention of disease
- Accessing resources for repositioning and repurposing of therapeutics
- Working toward a patient and health care environment that addresses dissemination, implementation, and access to new treatments and health information
Goal 2: Enable All People to Contribute to and Benefit from Translational Science: People are at the center of our translational research efforts. They are instrumental in identifying and addressing biomedical needs and opportunities. NCATS needs to develop collaborations and partnerships with a diverse set of stakeholders to enable translation to happen more effectively and efficiently. We strive to incorporate Diversity, Equity, Inclusion, and Accessibility (DEIA) and belonging into all aspects of our work and our workforce.
Goal 2 includes, but is not limited to, the following themes:
- Examining and addressing the potential for DEIA in translational science research and operations
- Including the patient as a partner in translational science
- Broadening community engagement to meet people where they are and create more inclusive and trustworthy approaches that engage the community and participants throughout the lifecycle of the study
- Addressing challenges in research and health disparities, including sex/gender, underserved populations, and issues across the life span
- Realizing the value of and working to increase the diversity and skill sets of the translational science workforce throughout career development
- Conveying the value of DEIA in translational science education and training
Goal 3: Accelerate Translation by Addressing Both Scientific and Operational Challenges: Innovation, creativity and technology can aid and accelerate translational science and research efforts, including identification of new opportunities to pursue. Teamwork and collaboration need to be recognized and supported as a critical aspect of effective and efficient translation.
Goal 3 includes, but is not limited to, the following themes:
- Applying data science to improve public health
- Developing strategies to utilize real-world data to enable new insights into health and our health systems
- Leveraging platform technologies to efficiently address public health needs
- Repurposing and multi-purposing resources
- Developing scalable and efficient solutions
- Supporting scientific and operational efficiencies to remove, reduce, or bypass barriers to translation
- Using innovation in prevention and diagnostic technologies
- Supporting efficient translation through team science, collaborations, partnerships, and networks
Goal 4: Advance Research and Operations that Cut Across Translational Science: These priorities span the different elements of the vision and cut across all parts of the mission of NCATS. The examples listed below span a number of preclinical and clinical areas that are key to bridging translational research roadblocks successfully.
Goal 4 includes, but is not limited to, the following themes:
- Supporting approaches to building team science
- Developing effective partnerships: cross-sector, cross-agency, and cross-Center collaborations
- Building relationships in local, regional, national, and global settings
- Supporting data sharing
- Repurposing of resources and infrastructure
- Supporting biostatistics, informatics, and other aspects of data science to inform design and implementation of studies
- Enabling open science
- Expanding outreach and education about translational science and its principles
- Identifying scientific policy barriers in translational science
- Considering preclinical and clinical structural and organizational barriers inhibiting translation
Goal 5: Work Together as Stewards for Advancing Translational Science: Stewardship is a value that supports responsible planning and management of resources. NCATS applies stewardship for both operational and scientific activities and efforts in order to responsibly use funds, resources, and people toward support of translational science.
Goal 5 includes, but is not limited to, the following themes:
- Addressing efficiencies and alignment with law, policy, and regulation in conducting/supporting translational science through our different business areas (grants, intramural, intellectual property and technology transfer, privacy, etc.)
- Setting priorities and sunsetting activities in a transparent manner (inclusive of future planning such as programmatic and budgetary operations planning)
- Supporting rigor and reproducibility
- Identifying measures of impact for translational science
- Communicating NCATS' successes
- Considering ethical issues in translational science
- Streamlining and coordinating internal processes and procedures and internal priorities and efforts



