Therapeutics for Rare and Neglected Diseases (TRND)
TRND program supports preclinical development of therapeutic candidates intended to treat rare or neglected disorders, with the goal of enabling an Investigational New Drug (IND) application.
About TRND
More than 6,500 rare and neglected diseases have been identified, yet only about 250 treatments are available for these conditions. The limited numbers of patients can make gathering information and designing drug studies difficult. As a result, scientists often know little about the symptoms and biology of these conditions. Also, some private companies may find it difficult to justify the cost of developing drugs for such small rare disease markets.
The Therapeutics for Rare and Neglected Diseases (TRND) program is designed to overcome these challenges. Its mission is to encourage and speed the development of new treatments for diseases with high unmet medical needs. TRND stimulates therapeutic development research collaborations among NIH and academic scientists, nonprofit organizations, and pharmaceutical and biotechnology companies working on rare and neglected illnesses. The program provides expertise and resources, working with research partners to move therapeutics through preclinical testing, including plans for clinical trials and submission of an IND application to the U.S. Food and Drug Administration (FDA). These efforts effectively “de-risk” therapeutic candidates and make them more attractive for adoption by outside business partners.
TRND Program Goals
The goal of the TRND program is to encourage and speed the development of new treatments for rare and neglected diseases. The program is designed to advance the entire field of therapeutic development by supporting scientific and technological innovations to improve success rates in the crucial preclinical stage of development.
TRND closes the gap that often exists between a basic research discovery and testing of new drugs in humans. That work includes the optimization and preclinical testing of therapies, with the goal to generate sufficient-quality data to support successful IND applications to the FDA and first-in-human studies in limited situations. Therapeutic clinical candidates that reach this stage should be attractive to biotechnology and pharmaceutical companies to take into clinical development.
TRND News
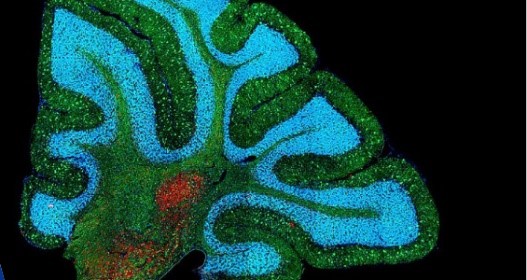
NCATS Enables a Drug’s Path to FDA Approval for Duchenne Muscular Dystrophy
March 15, 2024 - NCATS News
- Our Impact on Drug Discovery and Development
- Our Impact on Rare Diseases
- Therapeutics for Rare and Neglected Diseases (TRND)
NCATS scientists conducted laboratory studies and provided guidance in the development of a drug for the rare muscle disease Duchenne muscular dystrophy.
Propelling Cures With Genetics Research: Interview With Joni L. Rutter, PhD, Director of the National Center for Advancing Translational Sciences at NIH
December 14, 2023 - Grantee/Partner News
- 3-D Tissue Bioprinting
- Bespoke Gene Therapy Consortium (BGTC)
- Clinical and Translational Science Awards (CTSA) Program
- New Therapeutic Uses (NTU)
- Platform Vector Gene Therapy (PaVe-GT)
- Rare Diseases Clinical Research Network (RDCRN)
- Somatic Cell Genome Editing (SCGE)
- Therapeutics for Rare and Neglected Diseases (TRND)
- Tissue Chip for Drug Screening
New 3-D Models Offer Insights Into the Biology of a Rare, Devastating Neurological Disease
July 28, 2023 - NCATS News
- 3-D Tissue Bioprinting
- Therapeutics for Rare and Neglected Diseases (TRND)
NCATS scientists created human cell–based models of a rare disorder, NGLY1 deficiency, to learn more about the disease’s development and biology.
Blood Pressure Drug Could Treat Lebers
April 4, 2023 - Media Coverage
- Therapeutics for Rare and Neglected Diseases (TRND)
Related Research
Clinical Trial Readiness for Rare Diseases, Disorders and Syndromes
Through this program, we offer grants for projects focused on collecting data needed to advance promising therapies and diagnostics for rare diseases toward clinical trials.

Rare Diseases Clinical Research Network
We oversee this NIH-wide grant program that supports medical research on over 200 rare diseases through clinical studies.
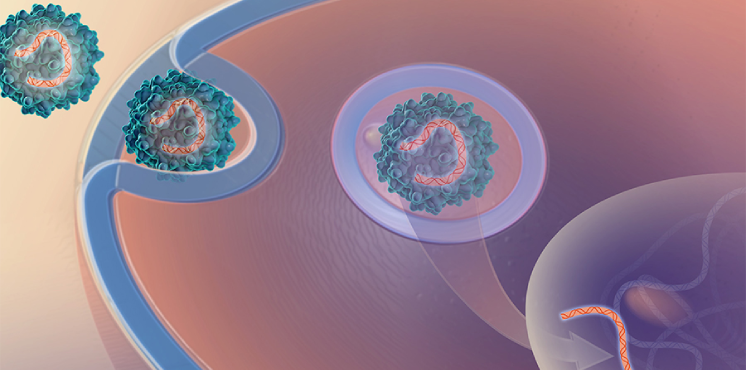
Platform Vector Gene Therapy (PaVe-GT)
Our pilot project is testing the feasibility of increasing the efficiency of gene therapy clinical trials by using the same gene delivery system and manufacturing methods for multiple gene therapies.


