NCATS, Duke Scientists Show Compound’s Novel Effects on Key Brain Chemical in Drug Addiction
March 3, 2022
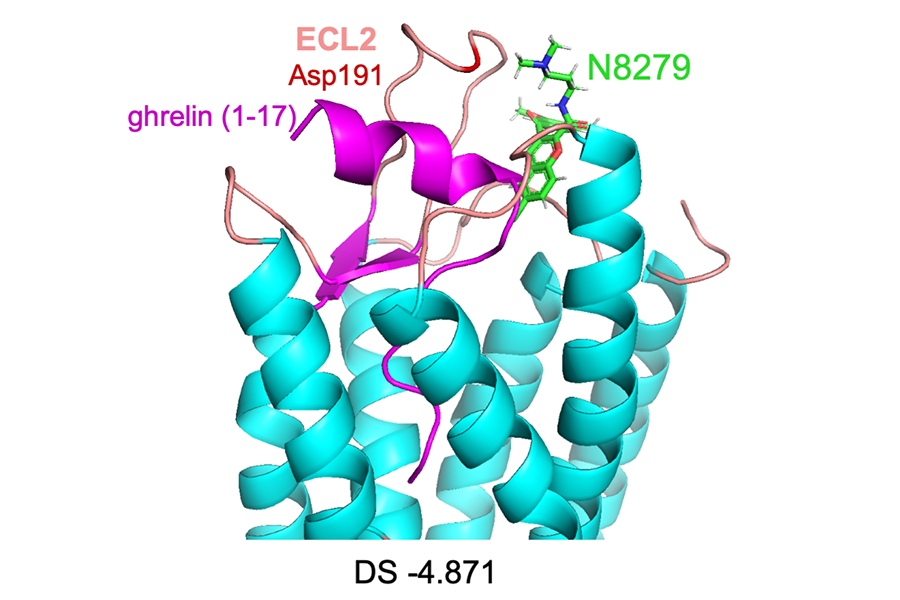
Scientists at NCATS and Duke University have discovered a novel compound that rapidly enters the brain and restores abnormal dopamine levels back to normal. The compound works by modulating the activity of the receptor, an entry point on a cell, for ghrelin, a hormone that affects the brain and other areas in the body. Here, a computer-generated model shows the compound, N8279 (green), sitting on the hormone (ghrelin, purple) and docked in the ghrelin receptor GHSR1a. (Joshua Gross, Ph.D., and Yang Zhou, Ph.D., Duke University)
Compound helps restore the brain’s normal dopamine levels, offering a potential approach to help treat addiction, Parkinson’s and age-related brain diseases.
Scientists at NCATS and Duke University have discovered a compound that can restore the brain’s normal amount of dopamine, a key chemical that fuels addictive behavior. Their studies in both cells and mice suggest a possible new treatment approach for drug addiction. This work eventually could have implications for treating and preventing other dopamine-related conditions, including such disorders as Parkinson’s disease and Alzheimer’s disease.
A collaborative team led by NCATS translational scientist Juan Marugan, Ph.D., and Duke University’s Lawrence Barak, M.D., Ph.D., and Marc Caron, Ph.D., reported these findings March 3 in the Proceedings of the National Academy of Sciences.
Activities that can produce pleasure or reward raise dopamine levels in the brain. The release of dopamine from brain cells is controlled in part by ghrelin, a stomach hormone that also has broad effects in the brain and other areas of the body. Scientists have looked for a way to harness ghrelin activity to control dopamine and possibly reduce the addictive effects of drugs. But ghrelin also is involved in releasing growth hormone, increasing hunger and controlling blood sugar. As a result, blocking its activity can bring unwanted side effects.
One possible way to regulate dopamine levels is through the ghrelin receptor. Many drugs work by either blocking or turning on a cell’s receptor, which is a chemical entry point on the cell.
“This ghrelin receptor can control the levels of dopamine in the brain and plays an important role in addiction,” said Marugan. “The receptor is considered one of the best targets for drug intervention.”
When the ghrelin receptor is turned on, it sends a chemical signal to brain cells by interacting with a pair of proteins, beta-arrestin and G protein. Beta-arrestin promotes the release of dopamine. When beta-arrestin is too active, the brain eventually produces too much dopamine. NCATS researchers used their expertise in drug screening to search through roughly 40,000 compounds to test their ability to interfere with beta-arrestin activity. They identified compounds that blocked beta-arrestin but also turned on the G protein’s activity.
“We found compounds that activate the receptor in a selective manner,” Marugan said. “Until now, it wasn’t clear how to do it. It’s a new compound that works in a fundamentally different way than all previously studied compounds. It’s able to reach the brain and is considered a good potential candidate for development.”
He noted that the compound, N8279, was not one of the compounds originally tested; it resulted from a chemical reaction that happened when testing a different compound that did not work.
“This type of receptor can send chemical signals that produce broadly different physiological and behavioral effects,” said Duke postdoctoral fellow Joshua Gross, Ph.D., a co-author. “We thought if a drug could either turn on or halt these signals through a specific pathway, we might be able to more selectively develop drugs. This strategy could lead to a more effective drug with fewer side effects.”
The Duke team showed that the compound could reduce dopamine-related behaviors in two different mouse models. In one experiment, the researchers found that the compound reduced the effects of cocaine on dopamine levels and calmed the animals’ hyperactive behavior. In a separate model, scientists shut down a key dopamine-related mouse gene, and again, the compound helped return normal behavior.
The scientists suggest that this treatment approach also can be applied to other disorders and conditions. The Duke group is planning to use their understanding of how the compound works to study its potential use in Parkinson’s and Alzheimer’s diseases, which are related to abnormal amounts of dopamine. In Parkinson’s disease, for example, the death of dopamine-producing brain cells leads to movement difficulties.
“We started on this narrow problem of drug addiction, and now we think this compound could be a key to understanding how to protect brain cells,” Barak said, adding that the compound might lead to a drug that could eventually help delay symptoms of neurodegenerative diseases or perhaps aging.
The researchers consider N8279 just a start. Marugan and his NCATS colleagues continue to modify the compound. They are creating newer versions that could be more selective in their effects on beta-arrestin and dopamine function while also attempting to improve how the body and brain use them. Duke scientists also are using different models to test the effects of these compounds, including their effects on behavior.


