Mount Sinai, NCATS Scientists Uncover a Potential New Path Against Neurological Disease
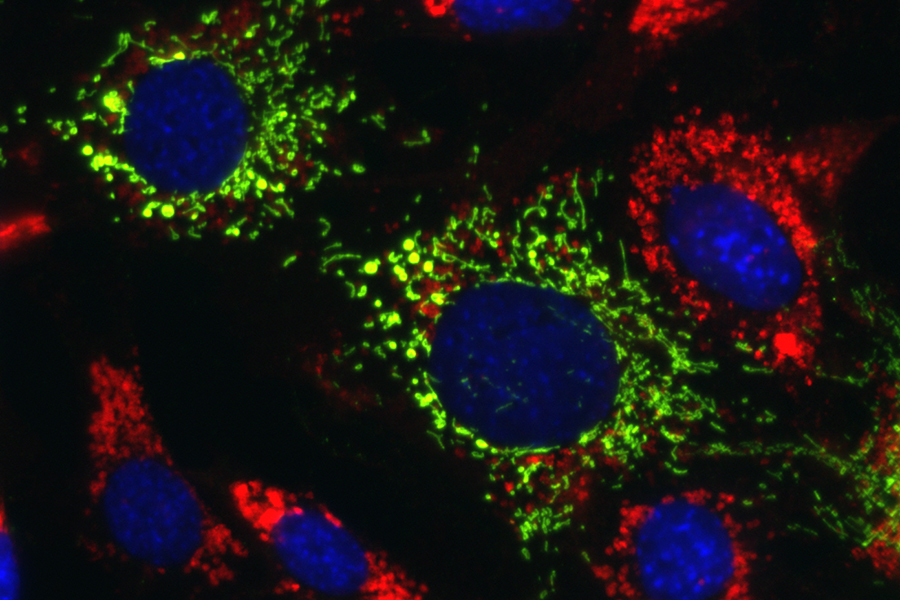
Researchers at the Icahn School of Medicine at Mount Sinai in New York and NCATS identified compounds that reversed the effects of several neurodegenerative diseases called lysosomal storage disorders (LSDs) in patient cells and mice. LSDs are characterized by genetic defects that prevent the cell’s lysosomes from breaking down and recycling fats, sugars and proteins, which can accumulate in the liver and brain. This accumulation eventually causes a malfunction in the energy-producing mitochondria. The compounds increased the activity of TRAP1, a protein that helps the mitochondria work properly. Here, lipid-filled lysosomes (red) from Niemann-Pick disease type C1 are normalized when mitochondrial TRAP1 (green) is activated. (Ioannou lab, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai)
September 8, 2022
Researchers at the Icahn School of Medicine at Mount Sinai in New York and NCATS reversed the effects of several life-threatening, inherited neurodegenerative diseases called lysosomal storage disorders (LSDs) in patient cells and mice. LSDs are characterized by genetic defects that prevent the cell’s lysosomes from breaking down and recycling fats, sugars and proteins, which can accumulate in organs, including the liver and brain. This accumulation eventually causes a malfunction in the energy-producing mitochondria, leading to further damage in these organs.
A team led by Mount Sinai’s Yiannis Ioannou, Ph.D., and NCATS translational scientist Juan Marugan, Ph.D., restored the proper function of both the mitochondria and lysosomes. The researchers suggest the findings could have implications for other neurodegenerative diseases that have similar underlying causes. They reported their results in iScience.
The researchers identified novel compounds that increased the activity of TRAP1, a protein that helps the mitochondria function properly.
“Researchers have always looked for drugs that could recover the function of lysosomes to try to impact lysosomal storage diseases,” Marugan said. “This is a new approach to these diseases. Increasing TRAP1 activity promoted mitochondrial protein folding and facilitated the cell’s proper balance. These first-in-class molecules activate TRAP1 in the mitochondria and reduce storage in lysosomal storage diseases.”
“Our data demonstrate that mitochondrial TRAP1 is a potential novel therapeutic target for multiple disorders that affect the central nervous system,” Ioannou said.
The Mount Sinai and NCATS teams began collaborating several years ago on a project involving the LSD Niemann-Pick disease type C1 (NPC1), a rare neurological disease.
Ioannou and his colleagues developed a test to measure the effect of compounds on NPC1. Marugan and his NCATS team subsequently used the test with NCATS’ high-throughput screening facilities to rapidly sift through thousands of compounds. They discovered that some compounds that activated TRAP1 made the mitochondria work properly again and restarted the lysosome’s recycling ability, helping reduce fats in the lysosomes and in cells. The researchers chemically improved the compounds that appeared to work best and tested them further.
The collaborative team showed that increasing the activity of TRAP1 in cells from people with NPC1 could correct their lipid storage disorder and restore normal cholesterol levels. In addition, increasing TRAP1 activity in cells from patients with other LSDs — such as Fabry, Farber and Wolman diseases — also corrected their respective lipid storage. In subsequent studies in mouse models of Fabry disease, the Mount Sinai researchers found similar results. Heightened TRAP1 activity in the animals resulted in a reduction in the abnormal amounts of lipids stored in the kidney, liver and heart.
The researchers discovered that TRAP1 initiated a “cross-talk” between mitochondria and lysosomes, which, they determined, was important to restoring the internal cellular balance.
“What is surprising is that TRAP1, when activated, initiates a cascade that leads to restoration of normal lysosomal function in lysosomal storage diseases,” Ioannou pointed out. “Keep in mind that the defect causing each lysosomal disease is still present, so this cross-talk bypasses the genetic defect.”
The scientists would like to understand more about how the compounds can reverse characteristics of the lysosomal storage diseases, which will be instrumental in developing potential drug treatments. They also plan to continue to refine these compounds and examine their effects in various models, including their ability to affect more common neurological disorders, such as Alzheimer’s disease and Parkinson’s disease.
“It’s known that similar dysfunctions in cell recycling and mitochondria health occur in other neurodegenerative disorders, such as Parkinson’s, amyotrophic lateral sclerosis and Alzheimer’s,” Marugan noted. “We believe that this approach could have therapeutic benefits for more mainstream disorders, as well.”
NIH…Turning Discovery Into Health®


