Stopping Metastasis in Its Tracks: New 3-D Cell Model Enables Closer Look at Cancer Progression
For hundreds of years, scientists and physicians have struggled to fight cancer. In the past half-century, the technological advances of chemotherapy and radiation therapy have enabled doctors to shrink patients’ tumors, but all too often, the tumors return and spread to other organs by way of a deadly process called metastasis. Until scientists can better understand and model this disease process, a cure for cancer seems unlikely.
This challenge inspired Ernst Lengyel, M.D., Ph.D., department chair and Arthur L. and Lee G. Herbst Professor of Obstetrics and Gynecology at the University of Chicago; and Hilary Kenny, Ph.D., an assistant professor in the department, to contact NCATS. Lengyel and Kenny’s team had developed a new way to study ovarian cancer metastasis and potential treatments using a 3-D model.
Historically, scientists have developed disease models by growing cells in a single layer in a laboratory. This monolayer approach is easy to construct but does not represent the complexities of the human body. As a result, many compounds that seem effective in a monolayer culture on plastic do not pass animal and human studies for safety and effectiveness. To improve the odds of success, Lengyel and Kenny’s team constructed a 3-D model of ovarian cancer metastasis using cells from patients. The team needed to optimize the model for high-throughput screening, which would enable the researchers to test many different compounds at varied concentrations at a much higher rate than could be done manually.
For this next step, Lengyel approached NCATS’ Ajit Jadhav via NIH’s Molecular Libraries Program and established a collaboration that also included NCATS scientists Marc Ferrer, Ph.D.; Madhu Lal-Nag, Ph.D.; Min Shen, Ph.D.; and Matt Boxer, Ph.D. The team worked together to create, adapt and validate an innovative 3-D cell model used to identify small molecules that can prevent cancer cells from spreading to new sites in the body. The new model includes connective tissue cells and the extracellular matrix (a collection of supportive molecules outside cells), creating a more lifelike simulation of the human body environment. It represents an exciting advance in research technology, and the high-throughput screening results, published in the Feb. 5, 2015, issue of Nature Communications, illustrate the power of this scientific collaboration.
Getting to the Root of the Problem
Metastasis is a three-step process. First, cells from the primary cancer site travel to a new area of the body. Next, the cells latch on to healthy cells. Finally, the cancer cells invade those healthy tissues. This process of cancer cell migration, adhesion and invasion — not the original tumor — is the root cause of many patient deaths. A drug that prevents one or more of these stages of metastasis could effectively stop this deadly process in its tracks.
To advance toward this ultimate goal, the NCATS-University of Chicago team first adapted and miniaturized a 3-D ovarian cancer cell culture model so they could use it in the high-throughput robotic screening system at NCATS. Just a few of the 2,420 compounds screened in the assay (test), including one called beta-escin, demonstrated an ability to prevent all three metastatic stages both in vitro (in an artificial environment) and in vivo (in whole, living organisms). Beta-escin is a naturally occurring substance derived from the seed of the horse chestnut tree and is commonly used to improve chronic venous insufficiency, a condition in which veins cannot pump enough blood back to the heart.
“Beta-escin was a very surprising finding,” Lengyel said. “You can buy it online. It is currently used to treat vein disease, and its anti-metastatic effect was unexpected.” Although beta-escin has promise, Lengyel explained that it has not yet been validated in human studies and should not be considered a cure. “It’s a promising lead to pursue,” he emphasized.
Innovation in 3-D Modeling
In addition to the encouraging findings from the compound screening, this collaborative project yielded another important advance: the cell culture model itself. The new 3-D model has many advantages over its monolayer 2-D predecessor, and the compounds identified using the new model are more likely to succeed in in vivo trials compared with compounds selected from 2-D screens. Unlike monolayer models, the 3-D approach can reproduce cellular and genetic tumor variations, complex tissue architecture, and interactions within the tumor microenvironment.
“The 3-D model we developed is a more accurate representation of cancer cells in the human body compared to 2-D models,” Lal-Nag explained. “This approach improves our ability to find drug candidates that are more likely to be successful.”
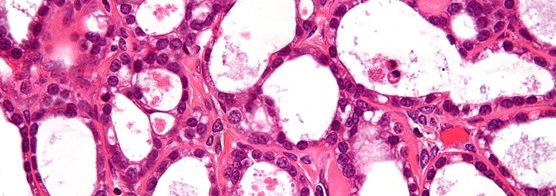
Very high magnification micrograph of an ovarian clear cell carcinoma. (Credit: http://bit.ly/1GXE2mc. Copyright: http://bit.ly/1fd019p)
By replacing generic cell lines with actual cells from ovarian cancer patients, the researchers introduced an element of precision medicine into the model. This specialized technique is particularly important for cancer treatments, for which the effectiveness of drugs varies widely from patient to patient. A personalized approach could reduce guesswork and unnecessary treatments.
“If we can take cells from patients and develop assays with those cells, the drugs we develop should work for that patient,” said Ferrer.
Applications Beyond Cancer
Although the team focused on ovarian cancer to demonstrate the power of the new 3-D model, this technology could advance translational research for diseases other than cancer. “It’s a general technology that translates to many different kinds of therapeutic approaches,” Jadhav said.
The potential for the new model also extends beyond therapeutic development: Its rich environment may offer basic scientists a new way of studying the underlying mechanisms of cancer and its progression. “The idea of migration, adhesion and invasion is fundamental to biology,” Jadhav said. “This could become a research tool for studying metastasis at the basic science level.”
The new model represents a different way of thinking about translational research. “Sometimes people think these type of assays are not possible,” Ferrer said. “I’m hoping this paper will change people’s minds — that the community will see this work and begin thinking beyond what’s usually done. We like it when people come to us with blue-sky ideas.”
Broadening the Scope
In the next phase of the project, the researchers will use the 3-D model to screen 400,000 additional compounds from another library. Validated compounds from this screen will be modified using medicinal chemistry to maximize strength and minimize toxicity before testing in animals.
“We hope there will be categories of compounds that act by different mechanisms, giving us an opportunity to explore these different classes of compounds,” Jadhav said.
This project exemplifies the power of collaboration for the benefit of scientists and patients alike. “That’s a common theme with NCATS projects,” Jadhav explained. “The passion that other investigators have for pursuing novel ideas is matched with the passion we have toward the same shared goal.”
“This advance would not have been possible without NCATS,” Lengyel added. “You would think that working with the government would be difficult, but this was seamless and a lot of fun. We are honored to work with such great scientists.”
Posted May 2015


