Our Impact on Research Operations
We cut through operational barriers so research studies can deliver answers faster.
Starting Studies Sooner
There are many steps that happen before new research projects can start. They can include negotiating agreements, building new infrastructure and bringing in the right expertise. Each step takes time and, as a result, slows start times and ultimately the results.
We have made these and other steps in the process faster and more efficient – shaving weeks and months off of project timelines. Our solutions include agreement templates that lay out the basic rules in advance, so collaborators can more quickly concur on the details. We also have developed research resources like chemical libraries and large clinical research networks that easily pivot to new research opportunities.
Because we use these approaches, we were ready – and stand ready – to respond quickly to public health emergencies. During COVID-19, these approaches kick-started therapeutic development efforts, real-world data sharing and major clinical trials. We share what we’ve developed and learned to help others speed their research efforts.
Impact Stories
National Pharmaceutical Collection (NPC)
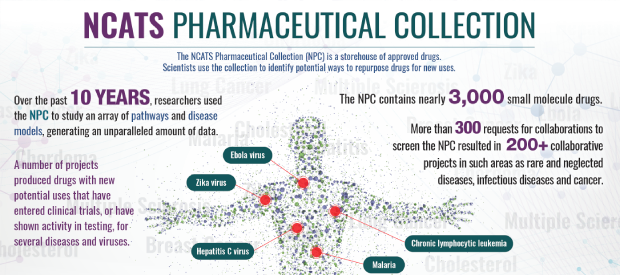
The NCATS Pharmaceutical Collection is a comprehensive, publicly accessible collection of approved molecular entities for high-throughput screening that provides a valuable resource for both validating new models of disease and better understanding the molecular basis of diseases and interventions.
How NCATS Compound Management Speeds New Therapies
Kelli Wilson, Ph.D., director of Compound Management at NCATS, explains how the NCATS Compound Management group supports researchers via compound storage, registration, shipping and reformatting.
(video length: 2:48)
Research Operations Activities
We develop programs and resources that overcome operational barriers slowing down research projects.

Clinical and Translational Science Awards (CTSA) Program
The CTSA Program addresses the development and implementation of national standards and best practices for translation, from basic discovery to clinical and community-engaged research.

Partner Forms and Model Agreements
We provide a full range of services to support NCATS technology development and partnership activities, including negotiating standard forms and model agreements between NCATS and outside parties.
Platform Vector Gene Therapy (PaVe-GT)
Our pilot project is testing the feasibility of increasing the efficiency of gene therapy clinical trials by using the same gene delivery system and manufacturing methods for multiple gene therapies.
Research Operations News

NIH Gene Therapy Team Reveals Its Path to FDA Orphan Drug and Rare Pediatric Disease Designations
March 29, 2023 - NCATS News
- Our Impact on Rare Diseases
- Our Impact on Research Operations
- Platform Vector Gene Therapy (PaVe-GT)
When NCATS received an Orphan Drug Designation (ODD) from the U.S. Food and Drug Administration (FDA), it marked a key accomplishment for researchers in the NIH's Platform Vector Gene Therapy (PaVe-GT) project.
Read ArticleNationwide IRB Reliance Agreement Aimed at Speeding Research Reaches 1,000 Signatories
August 3, 2022 - NCATS News
- Clinical and Translational Science Awards (CTSA) Program
- Our Impact on Research Operations
The Streamlined, Multisite, Accelerated Resources for Trials (SMART) Institutional Review Board (IRB) agreement has reached 1,000 participating sites, making it one of the largest medical research
EPA Holds Meeting on TSCA New Chemicals Collaborative Research Program
April 22, 2022 - Media Coverage
- Our Impact on Research Operations
High-Throughput Screening Helps Advance Melanoma Therapy
March 23, 2022 - NCATS News
- Our Impact on Research Operations
Finding the right compounds for new uses in treating a disease or improving a therapeutic approach can be time consuming.



