Incorporating Stable Glycosides into Novel Small Molecule Scaffolds
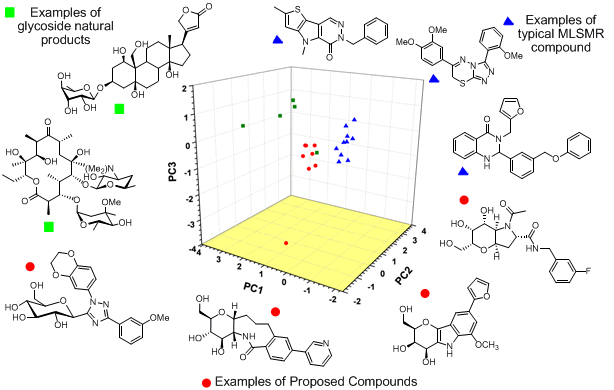
Modern insights into the relationship between small molecule structure and biological function have compelled chemists and biologists to shed predetermined notions regarding the make-up of screening libraries and pressed for the inclusion of non-traditional small molecules. One consistency, during the evolving strategies associated with small molecule design, is the mimicry of the molecules of life. Historically, molecules designed to imitate peptides and nucleic acids have strong precedence in screening libraries and as molecular probes and drugs. Conversely, carbohydrates and carbohydrate mimetics have lagged behind in their development as library components for high-throughput screens. Glycosides have substantial structural uniqueness in relation to the other three classes of biomolecules, and in comparison to typical small molecules found in screening libraries. Based upon these advantages and the lack of prior art in this area, we are designing and constructing small molecules that incorporate stable C-glycoside based small molecules for primary screening efforts. Examples of these agents and their comparison to natural products and typical library members via a 3D chemical diversity plot is shown below.
Lead Collaborator
National Cancer Institute
Craig Thomas, Ph.D.
Public Health Impact
This study presents several novel chemotypes for small molecule library development based upon stable C-glycoside monomers.
Publications
Hajduk PJ, Galloway WR, Spring DR. Drug discovery: A question of library design. Nature, 2011;470:42-43.
Rishton GM. Nonleadlikeness and lead likeness in biochemical screening. Drug Discov Today, 2003;8(2):86-96.
Outcomes
In progress.


