NCATS, Karolinska Institutet Scientists Attack Cancer’s Defenses
February 24, 2018
Scientists from NCATS and Sweden’s Karolinska Institutet have developed a potential new approach to fighting cancer by breaking down a defense system used by cancer cells.
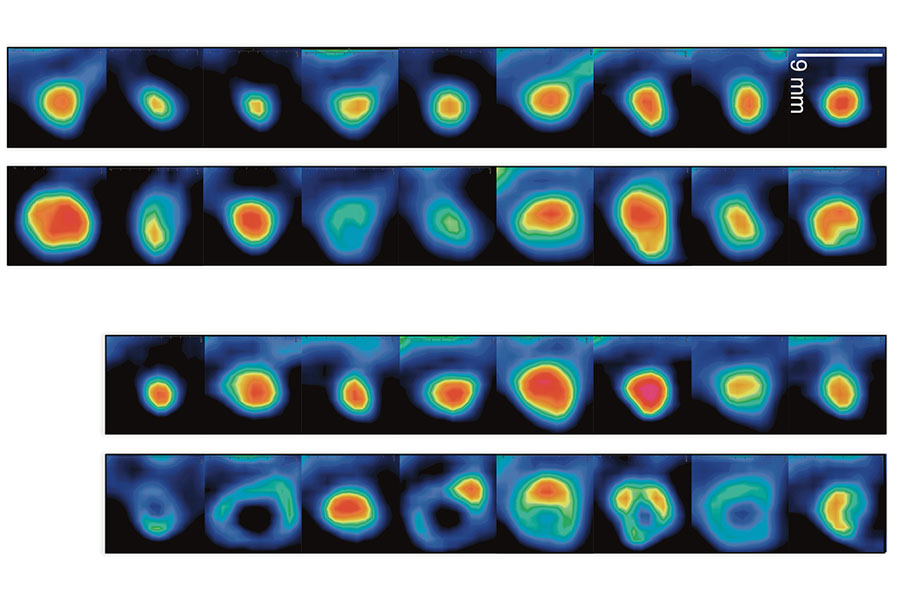
Scientists from NCATS and Sweden’s Karolinska Institutet identified a compound, TRi-1, that can kill cancer cells and reduce tumor growth. The top two rows of images show tumors treated with a different compound that has no effect at the beginning of treatment (top) and at day three (bottom). The lower two rows show tumors treated with TRi-1, again at the beginning of treatment (top) and at day three (bottom). The images in the bottom row reflect the compound’s effects on reducing tumors. (Reprinted with permission from Stafford et al., Sci. Transl. Med 10, eaaf7444 (2018).)
The defense system involves an enzyme, thioredoxin reductase 1 (TrxR1), which supports cancer cell survival. The research team identified a compound, TRi-1, that stops the activity of the enzyme without causing unwanted side effects, which can be common with existing chemotherapy drugs.
When applied to breast and head and neck cancers in mice, TRi-1 killed cancer cells and reduced tumor growth, yet appeared to leave healthy cells and tissues alone. These results, reported Feb. 14, 2018, in Science Translational Medicine, suggest that interfering with cancer cells’ ability to protect themselves could be a useful strategy against many types of cancer.
“There haven’t been any drugs specifically designed to target TrxR1,” said study co-author Anton Simeonov, Ph.D., NCATS scientific director. “These findings provide the first evidence of promising starting points to develop cancer drugs that work against only this enzyme.”
Some existing thioredoxin reductase inhibitors are used to treat diseases such as rheumatoid arthritis and leukemia, and other inhibitors are in clinical testing for other disorders, but no drugs currently on the market target this enzyme as specifically as the new compound does
In 2010, co-author Elias Arnér, M.D., Ph.D., professor of biochemistry at Karolinska Institutet, approached Simeonov and his NIH team to collaborate in developing inhibitors against the enzyme, which Arnér had been studying for more than a decade.
The team needed to find specific compounds against TrxR1 that didn’t cause side effects. The researchers evaluated nearly 400,000 compounds, testing various amounts for their effectiveness in blocking enzyme activity. NCATS provided access to the compound library and the expertise and technology to screen the large number of compounds, in addition to expertise in developing needed assays (tests) and expertise in medicinal chemistry to determine the most likely kinds of molecules that could block the enzyme alone. This allowed the researchers to identify candidate compounds and — through further testing and refining — move them along the translational path to determine which were most effective against cancer cells and in animal models of cancer.
“To avoid damaging normal cells, we had to ensure the compounds didn’t also inhibit another enzyme found in cells, which is similar in structure to TrxR1,” Simeonov said. “This was a key test we ran first at NCATS and later at Karolinska Institutet to select the most promising molecules that showed more specific activity against TrxR1.”
Through another series of tests and analyses, the scientists narrowed the list to 53 candidate compounds — including those that worked against TrxR1 and molecules with similar structures — and evaluated their potential as drugs. When the team studied these compounds for activity against cancer cells and in enzyme activity assays, two inhibitors — TRi-1 and TRi-2 — stood out.
The researchers examined the effects of TRi-1 and TRi-2 against TrxR1 in about 60 different cancer cell lines, and TRi-1 proved to be the most specific in blocking enzyme activity. The type of cancer did not seem to matter. In tests with mice, Arnér and his Karolinska Institutet colleagues showed that TRi-1 was also more effective than other candidate compounds in killing cancer cells and reducing tumor growth.
Although more testing of TRi-1 and similar compounds is needed in the laboratory and eventually in cancer patients to demonstrate safety and effectiveness, the published results suggest that TrxR1 inhibitors will likely be most effective when combined with other cancer therapies.
“These results are very promising because they raise the possibility of developing newer and hopefully more effective therapies for cancer patients,” Arnér said.


