NIH, Tuskegee Researchers Develop Potential New Type of Immunotherapy Across Cancers and Many Other Diseases
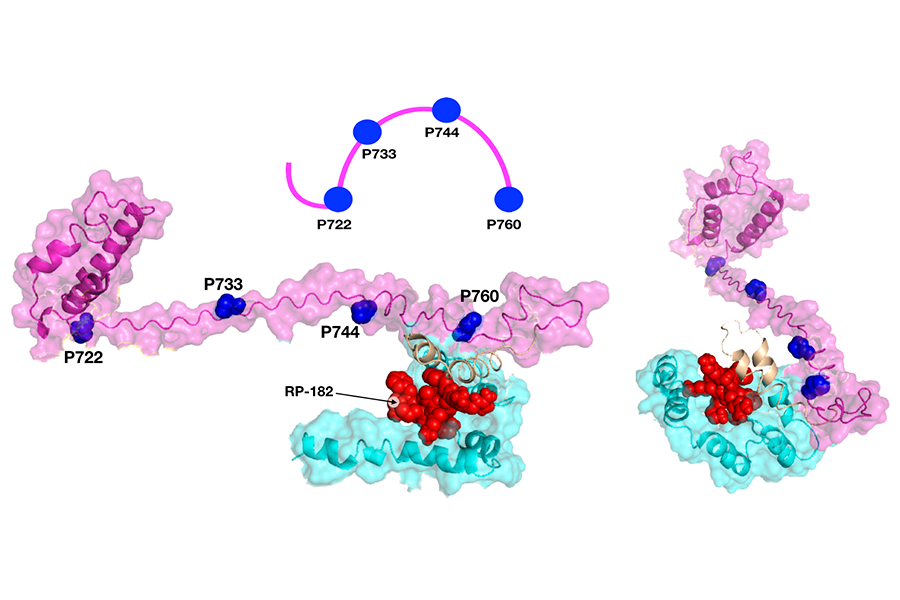
A model shows how a small protein, or peptide, called RP-182 (shown in red) is nestled inside a receptor, CD206, in two specific locations (shown in magenta and cyan). This alters the receptor’s shape and activates the receptor. (Jaynes et al., Sci. Transl. Med. 12 (2020): eaax6337)
February 26, 2020
In reprogramming immune cells to fight tumors, scientists take a page from an ancient playbook
Tumors can co-opt the body’s immune defenses to evade detection and boost cancer growth. Now, scientists at the National Institutes of Health (NIH) and Tuskegee University in Alabama suggest a potential new therapeutic strategy that employs the immune system against cancer. The team — led by researchers from NIH’s NCATS and National Cancer Institute (NCI) and the College of Agriculture, Environment and Nutrition Sciences at Tuskegee — has shown in laboratory experiments that a novel type of immune therapy can reprogram immune cells to kill cancer cells and halt tumor growth in several types of cancer. The scientists, who reported their findings in the February 12 issue of Science Translational Medicine, also showed the approach has potential for treating other diseases.
In recent years, advances in immunotherapy — which harnesses the body’s immune system to fight disease — have garnered headlines as a potentially powerful tool against cancer. But many cancers don’t respond to immunotherapies, and scientists have sought to develop new and more effective immunotherapy approaches.
“The field of immunotherapy holds immense promise and has already changed the cancer treatment landscape in immeasurable ways,” said NCATS Director Christopher P. Austin, M.D. “These new findings point to a potential new strategy for using the immune system against cancer and underscore the value of combining expertise in fields like cancer biology, pharmacology and medicinal chemistry in a collaborative effort to move a basic science observation forward.”
The work built on research conducted by biochemist Jesse Jaynes, Ph.D., at Tuskegee University. He was studying immune system–related peptides (small proteins) called host defense peptides, which are produced by immune cells, and their roles in infectious disease. These peptides punch holes in the cell membranes of bacteria, killing the bacteria. They also attract a type of immune cell called tumor-associated macrophages to tissue injury sites — but cancers also attract these macrophages for help escaping the immune system and to help the cancers grow.
Jaynes saw commonalities among these peptides in many species, and he and his colleagues suspected certain host defense peptides could have broader effects on activating the immune system, especially in cancers.
In the study, the researchers tapped into an ancient part of immune system defenses used by organisms to fend off bacteria, viruses and other foreign invaders. They discovered similarities among peptides that play a role in immune systems across organisms and over millions of years of evolution. This research also suggested that these similarities affected the behavior of immune cells, including tumor-associated macrophages. Macrophages are frequently the first line of defense against infection but, conversely, are also cells subverted by cancers.
Jaynes’ group, in collaboration with co-authors Udo Rudloff, M.D., Ph.D., a physician-scientist at NCI, and Juan Marugan, Ph.D., a team leader at NCATS, uncovered a receptor, a docking station called CD206 that one peptide uses to attach to the surface of macrophages.
“CD206 was likely an entry point on macrophages during evolution for different invading organisms, like bacteria and viruses,” Jaynes said. The team modified the peptide, creating a synthetic version, RP-182, which could bind more strongly to the CD206 receptor.
How the peptide acts and what it does
One goal of the work was to see if the peptide’s ability to attract macrophages would be useful in fighting cancer. Would it be possible to switch the macrophage’s cancer-promoting behavior back to a normal, antitumor, immune system response?
NCATS and NCI scientists conducted a series of studies to find out how CD206 works. They found that when RP-182 binds to CD206, the receptor structure changes, sending new chemical signals to reprogram a type of macrophage, M2, to begin killing — literally eating — cancer cells. The NCI group visualized the structural change under a special microscope, while NCATS scientists detailed how RP-182 attached to the receptor.
In tumors treated with RP-182, the researchers found that these reprogrammed M2 macrophages devour tumor cells. RP-182 converts M2 macrophages into another macrophage subtype, M1. They saw an increase in M1 macrophages, which consume bacteria, viruses and cancer cells.
The RP-182-receptor interaction also had additional anticancer effects. “In addition to converting the macrophages into cancer killers, we found that when RP-182 attached to and altered the CD206 receptor, this also activated an array of other immune cells that help against the cancer,” Marugan said.
When Rudloff and his colleagues at NCI gave RP-182 and chemotherapy or another immune therapy to mice with pancreatic cancer, those mice exhibited fewer signs of the disease. These results suggested the possibility of combining this type of therapy with other treatments, including other immunotherapies. The Tuskegee researchers tested RP-182 in additional animal models of cancer, showing that it was also effective against other cancer types and could have wide applicability.
“These kinds of peptides existed 400 million years ago,” said Rudloff. “We’ve connected an evolutionary process to an immune mechanism from which we can make a cancer drug. Our results could have broader implications for developing a new approach to immunotherapy.”
RP-182 could work in noncancer-tissue mouse models, too. In a lung fibrosis model, the researchers found it reduced lung damage driven by abnormal macrophage activity, and the animals’ conditions improved.
“It’s likely there are other examples like this that could provide exciting templates for new cancer drugs,” Rudloff said. “For our patients, our priority is to further develop RP-182 as a cancer drug and get it into the clinic. That’s still a while away.”


