Small Business Awards Propel the Development of a “One-of-a-Kind” Tissue Chip Technology
January 12, 2021
About 30% of drugs successfully tested in animals are toxic in humans, and another 60% of these candidate drugs fail to show efficacy in human trials. For neurological drugs, the translation rate is even lower.
With support from the National Center for Advancing Translational Sciences (NCATS) Small Business Innovation Research (SBIR) Program, scientists are solving this problem by creating new tissue chip technology that can predict the toxicity of candidate drugs in human organs. Tissue chip devices are designed to be accurate mimics of the structure and function of human organs, such as the lungs, liver, heart and nervous system. Researchers can use tissue chips to predict whether a candidate drug, vaccine or biologic agent is safe or toxic in humans in a faster and more effective way than current methods.
Lowry Curley, Ph.D., was a postdoctoral investigator working in the laboratory of Michael Moore, Ph.D., at Tulane University when they began developing a peripheral nerve tissue chip to examine ways to encourage nerve growth after spinal cord damage. Discussions with pharmaceutical drug developers led Curley and Moore to realize that their invention could also be a valuable new tool for testing the effects of drugs on peripheral nerves. With that in mind, the investigators founded AxoSim, Inc., in 2014, with the initial goal of developing a Nerve-on-a-Chip®, a three-dimensional platform incorporating live nerve cells that looks and acts like a real human nerve.
AxoSim, Inc., had already demonstrated that the Nerve-on-a-Chip® could be used to measure the speed and strength of electrical signals across the nerve when it received initial funding from NCATS’ Small Business Technology Transfer (STTR) program in 2016. This Phase I grant allowed AxoSim to demonstrate that the Nerve-on-a-Chip® could predict the effects of four chemotherapy drugs on peripheral nerves. AxoSim subsequently received an NCATS Phase II SBIR grant to ensure that the technology works and bring it to market.
By the end of the Phase I grant, AxoSim had its first industry customer. “With the dataset we generated from testing those four drugs, we were able to start discussing the platform with pharmaceutical companies. ...It showed the promise of this technology,” explained Curley. Before completing the Phase II grant, AxoSim had contracts with six pharmaceutical companies to use the Nerve-on-a-Chip® to test a wide range of cancer drugs. AxoSim also received a National Institute of Environmental Health Sciences (NIEHS) SBIR grant to test the effects of chemical toxins on the nerve. As a result of the proof-of-concept data generated through the research supported by NCATS’ STTR/SBIR funding, AxoSim raised seed funding from investors.
NCATS’ SBIR and STTR programs are important for the academic entrepreneur whose university “does not have the funding resources to start a company,” said Lili Portilla, M.P.A., Director of NCATS’ SBIR and STTR programs. “SBIR/STTR provides entrepreneurs in the life sciences that early-stage funding they need to get their ideas percolating and to a point where they can then leverage other funding to move the technology into the commercial space and translate them into the commercial ecosystem.”
According to Danilo Tagle, Ph.D., Associate Director for Special Initiatives, NCATS is encouraging U.S. small businesses with promising technologies, like AxoSim, to get their products “to market as soon as possible and make a tremendous impact in the field right away.”
A One-of-A-Kind Technology
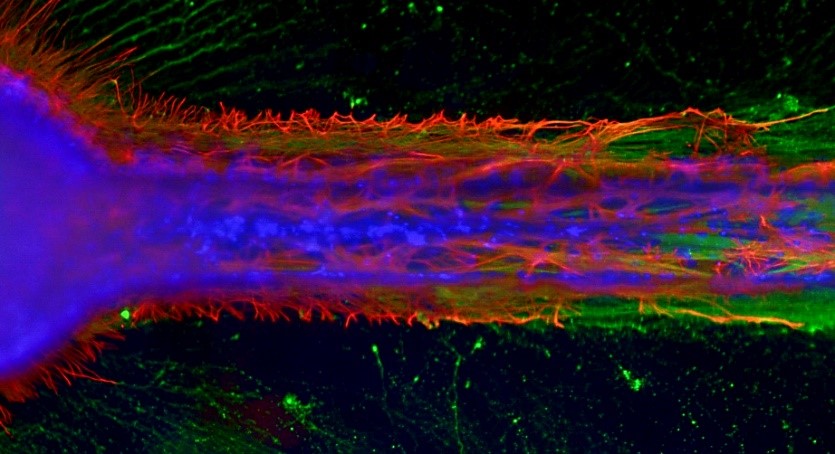
This in vitro mini-nerve fiber tract shows constrained axonal outgrowth (green) interacting with astrocytes (red), and includes migrating supportive cells (blue).
AxoSim’s Nerve-on-a-Chip® is seen as a model system and the first of its kind in the United States, according to Tagle. This technology has the unique advantage of showing nerve myelination, which is critical to the rapid transmission of electrical signals that allow the peripheral nervous system to communicate with the central nervous system. Myelin also is the part of the nerve that is destroyed by some cancer treatment drugs and many neurological diseases.
The unique translational potential of the Nerve-on-a-Chip® lies in its ability to replicate tests performed in the clinic. Patients experiencing neuropathy undergo tests to measure nerve conduction velocity (how fast signals move from one end of the nerve to the other) and the strength of those signals. Some patients also undergo biopsies so that the neurologist can examine the structure of the nerve for possible damage. Because the Nerve-on-a-Chip® mimics the structure and function of human tissue, this platform could be used to perform similar tests and to examine changes in the structure of the nerve. According to Portilla, this translational potential was a key factor in NCATS’ decision to support the development of the Nerve-on-a-Chip®.
Future Clinical Applications for the Nerve-on-a-Chip®
The Nerve-on-a-Chip® technology has a wide range of potential applications for clinical practice beyond nerve conduction studies and drug testing. One of these applications is testing nerve damage and pain in specific conditions, such as amyotrophic lateral sclerosis (ALS). Other applications include screening new pain therapeutics, testing the effects on the nervous system of compounds found in consumer and manufacturing products, and customizing therapies for individual patients (personalized medicine).
Personalized or “precision” medicine is an area of particular interest to AxoSim. “By using a patient’s own cells, we want to predict not only that a drug works but how it will work for every person. Precision medicine is already happening in cancer, and we really want to move in that direction,” said Curley.
Before health care providers can use the Nerve-on-a-Chip® in precision medicine, scientists will need to demonstrate that they can use patient-derived stem cells to grow peripheral nerve tissue and embed this tissue in a chip. Scientists also will need to show that specific diseases of the peripheral nerve can be represented in the Nerve-on-a-Chip®. Although the supporting technologies needed to accomplish these steps are not yet fully developed, Curley and his colleagues have used generic, patient-derived cell lines to grow nerve tissue on a small scale.
According to Curley, “Our biggest focus and challenge right now is adding more automation,” which will allow researchers to test more agents faster using the Nerve-on-a-Chip®. The NIEHS SBIR grant supports a partnership between AxoSim and the University of Central Florida to automate the Nerve-on-a-Chip®. Incorporating automation also is an important step toward creating a Nerve-on-a-Chip® kit-based product, which would allow broader use of the technology.
Advice for Would-Be Entrepreneurs
Curley has some sage advice for other biomedical entrepreneurs, particularly those in academia. “My main advice for people at universities who are preparing to spin out a discovery or technology to form a new enterprise is the importance of understanding your customers and listening to them.”
Curley encouraged both academic and nonacademic entrepreneurs to apply for NCATS SBIR/STTR grants—and to persevere if their first application is not successful. AxoSim, for example, had to resubmit its application for Phase I funding. “The first time we got really good feedback [from NCATS] that helped us prepare a stronger application for the second submission.”
Curley emphasized that the benefits of the NCATS Small Business Awards are not only financial. “They [NCATS] understand, they’ve got their finger on the pulse of what industry’s looking for” in responding to translational science bottlenecks and regulatory issues. The program is “really a bridge to other organizations—partners, collaborators, customers.”


