NIH-Funded Centers Look to Make Tissue Chips FDA-Ready Tools
July 25, 2024
NCATS has established new centers to strengthen the use of organ-on-a-chip, or tissue chip, technology to develop drugs and potentially reduce the need for animals in research.
NIH has awarded approximately $31 million over five years to support four Translational Centers for Microphysiological Systems (TraCe MPS) at the University of Washington, Seattle and the Icahn School of Medicine at Mount Sinai in New York; the University of Rochester; the University of Pittsburgh; and Texas A&M University, College Station and The University of Texas Medical Branch (UTMB), Galveston.
For more than a decade, NCATS has led a multifaceted research program to create and test tissue chips. Tissue chips are tiny, bioengineered 3-D models that use live cells to mimic the function and biology of tissues and organs. Researchers have studied the use of tissue chips to test drug toxicity, develop drugs, model diseases and design clinical trials. Tissue chips also have been sent to the International Space Station and brought back to help scientists better understand diseases and conditions linked to aging.
The TraCe MPS program is a partnership with the U.S. Food and Drug Administration (FDA). The FDA will work with each funded group to help it meet certain requirements for the chips to qualify as FDA-sanctioned drug development tools.
“The centers are the last step in moving tissue chips into widespread use,” said Danilo Tagle, Ph.D., director of the NCATS Office of Special Initiatives. “The use of animal models hasn’t had an especially good success rate in developing drugs. Currently, tissue chips are mostly considered complementary to the use of animals in research. This has already begun to change.”
In 2022, the FDA Modernization Act 2.0 allowed drug registration without using animals to test drug safety, including toxicology studies, if other risk assessment tools, such as tissue chips, are available. With tissue chips designated as drug development tools, the data they produce can be used alone as evidence in an Investigational New Drug (IND) application. The FDA requires that an IND be submitted before a drug can be tested in people.
Tagle sees such FDA recognition as key for industry to begin to use tissue chips more widely in drug development. “Qualifying tissue chips as FDA-certified drug development tools will give companies the impetus to use them, and perhaps less of a reason to have to use animals,” he said.
Investigators will need to show how the technology compares to the current animal model standard in drug development. “The funded teams must show the data from tissue chips are representative of humans and clinically relevant,” Tagle said.
New Projects Explore Liver, Kidney Diseases, Pregnancy and Barrier Function
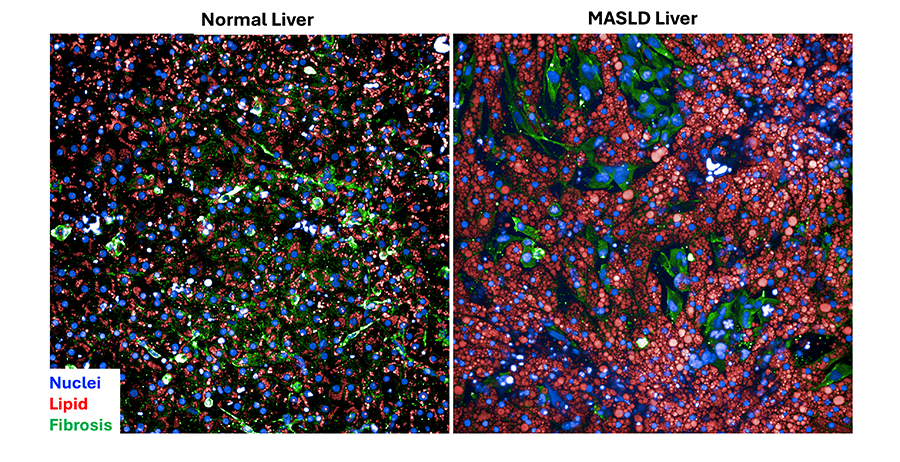
Lansing Taylor, Ph.D., and his colleagues at the University of Pittsburgh plan to study liver-on-a-chip models for metabolic dysfunction–associated steatotic liver disease (MASLD). MASLD is a condition in which too much fat builds up in the liver. It affects more than a quarter of the world’s population. Taylor’s team will show how the tissue chip models can be used to study drug clearance and toxicity in the liver, and for testing drugs and selecting patients for clinical trials. The scientists hope the liver chips will help them develop treatments for specific patient groups.
“Traditional drug discovery approaches haven’t really worked,” Taylor said. “Animal models don’t accurately reflect human pathophysiology for diseases like MASLD.”
The University of Rochester team, led by James McGrath, Ph.D., is working with Duke University researchers to develop tissue chip tools to better understand the roles of physiological barriers in disease. Some parts of the body, such as the gut and brain, have protective barriers to keep out harmful organisms and substances. Many of these barriers play important roles in infection and cancer.
Tissue chips could help the research team better study the role of the protective blood–brain barrier and develop drugs for central nervous system disorders. The team also will study other conditions, such as autoimmune disease and sepsis.
The lack of effective drugs to treat most kidney diseases and the need for accurate research models make the use of non-animal systems even more crucial, noted Jonathan Himmelfarb, M.D. Himmelfarb leads a group of investigators at the University of Washington and the Icahn School of Medicine at Mount Sinai. The group plans to assess the use of kidney tissue chip models in studying drug toxicity, the progression of kidney disease, and the effectiveness of therapies in both common and rare diseases.
“The funding award is the natural progression of work we’ve done with in vitro microphysiological systems to understand kidney disease biology and develop new therapies,” Himmelfarb said. “The new center allows us to show the strength and reproducibility of our models and enable others to have confidence in their uses as drug development tools.”
Scientists at UTMB and Texas A&M University will show how tissue chips that model pregnancy and women’s health can be used. The chips mimic healthy and diseased tissues inside the uterus. The team, led by UTMB’s Ramkumar Menon, Ph.D., will study the use of tissue chips as preclinical models to examine which drugs cross the placental barrier and can potentially harm the developing fetus. They plan to develop biomarkers to assess the response by the fetus and placenta together to therapeutics and will develop new therapies for pre-term birth.
Tagle called the use of tissue chips as drug development tools “a paradigm shift.”
“We’re moving away from standard practice to something new, more efficient, more predictive, accessible and perhaps more economical for investigators,” he said.
In addition to NCATS, the National Institute on Aging also is supporting the new centers.