Retooled Methotrexate Could Lead to New Therapies for Cancer, Autoimmune Disorders
February 2, 2024
Methotrexate is a decades-old mainstay in the treatment of blood cancers and rheumatoid arthritis. But the drug has downsides, including drug resistance and harsh side effects. An NCATS-led research team has designed a new way to build a better methotrexate. Their innovative approach could deliver less toxic versions and create new tools that lead to more treatments for cancer, autoimmune disorders and rare diseases.
“This research provides scientists new tools to study and unravel the enigmatic properties of methotrexate and similar compounds,” said James Inglese, Ph.D., director of NCATS’ Assay Development and Screening Technology laboratory and a corresponding author on a study describing the research.
Researchers have tried before to build a better version of the drug. Inglese and his translational science team of researchers at NCATS, the University of Oklahoma and the National Cancer Institute developed a different method.
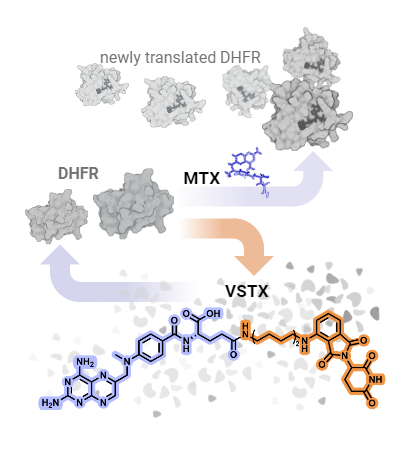
Methotrexate works by blocking a protein, dihydrofolate reductase (DHFR) enzyme, that plays a key role in helping cells multiply. In new work published in Cell Chemical Biology, NCATS scientist and study lead author Sandeep Rana, Ph.D., used knowledge of how methotrexate binds to DHFR to create compounds called PROTACs, or proteolysis targeting chimeras, that incorporate methotrexate.
PROTACs mark target proteins for attack. They hijack the cell’s natural waste disposal machinery to destroy a protein — in this case, DHFR. NCATS scientist and study coauthor Patricia K. Dranchak, Ph.D., paired the PROTAC development with the design of new assays to study the PROTACs’ cellular and biochemical properties.
The researchers tested one of the new PROTACs, called versortrexate, in several cancer cell lines. They found that it could break down DHFR while appearing less toxic, which might lead to fewer side effects. They also developed methotrexate-resistant cancer cell lines, showing that these PROTACs might be able to overcome such cells’ drug resistance. The researchers derived the name versortrexate from the Latin for “turn back” — in this context, to break down DHFR and turn it back into its chemical building blocks.
Methotrexate’s effects in the body are not well understood, Inglese pointed out, given that it hits more than one target in a cell. The new research allowed the team to learn more about how methotrexate works, including its toxic effects. The researchers also see versortrexate as a chemical tool to use to better understand DHFR activity. Versortrexate could also reveal how similar antifolate drugs work.
Compared with methotrexate, versortrexate is much less toxic, Inglese said. Versortrexate also has a very specific activity, which opens a range of possibilities for development. Combining versortrexate or other candidate drugs with a companion drug that inhibits another protein in the same or a different biochemical pathway might prove effective. Inglese noted that such PROTACs also could be tested for other disease types, perhaps even for rare metabolic diseases.
The methotrexate-based PROTACs are a different way to think about drug repurposing, or using old drugs in new ways, Inglese explained. “Molecules like methotrexate are incredibly well studied and highly developed,” he said. “That means there’s a lot of room to do other things with them. They can be scaffolds for the next generation of therapeutics or chemical probes.”