NCATS-Led Team Finds Potential Strategy to Fight Huntington’s Disease
January 11, 2018
A team of NIH scientists and collaborators has uncovered a new potential strategy against Huntington’s disease, an inherited, fatal neurodegenerative disorder that has no cure. In a study published recently by eLife, researchers at NIH’s National Center for Advancing Translational Sciences (NCATS), the University of Michigan and Baylor College of Medicine demonstrated that blocking the activity of an enzyme called PIP4Kγ reduced the effects of the disease in both cell and animal models. The findings indicate that inhibiting the enzyme triggers a normal-cell-“recycling” system that helps lessen the buildup of malformed, toxic proteins in brain cells that is associated with neurological problems.
In Huntington’s disease, a single faulty gene causes a progressive breakdown of nerve cells in the brain and severe physical and neurological damage leading to death. Early symptoms of Huntington’s disease, such as uncontrolled movements or difficulty focusing, typically begin in a person’s 30s or 40s; over time, people with the disorder lose the ability to move, speak and think. About 30,000 people in the U.S. have the disease.
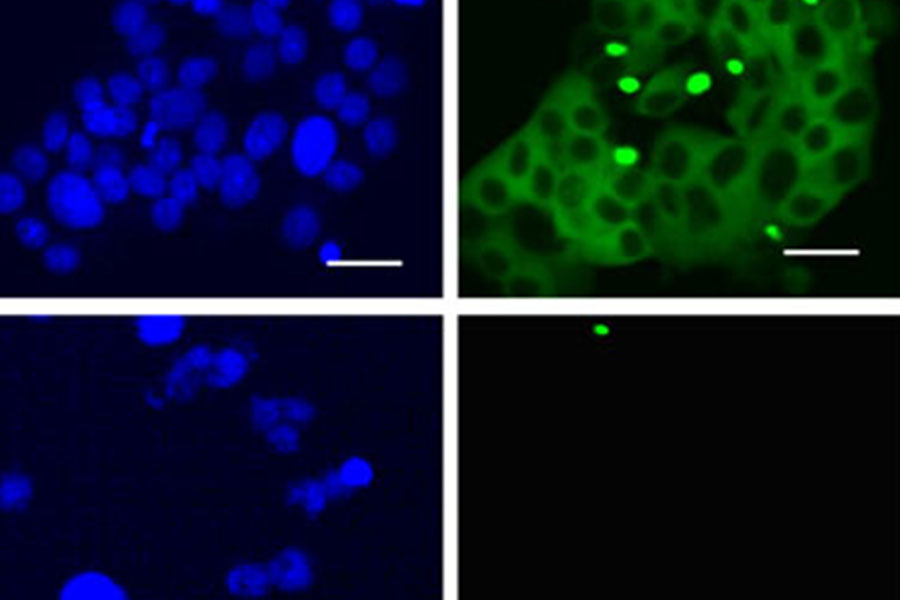
In a cell model of Huntington’s disease, the compound NCT-504 lowers the amount of toxic protein in the cell by inhibiting the enzyme PIP4Kγ. Here, the panels show cells before (top) and after treatment (bottom) with NCT-504. The right column indicates the abundance of the toxic protein. The cells after treatment with NCT-504 show a significant decrease in toxic protein accumulation.
A common characteristic among many neurodegenerative disorders is a breakdown in the cell’s ability to discard its cellular waste, a process called autophagy. In autophagy, damaged proteins and worn-out cellular components are delivered to the cell’s lysosome, a compartment where these components are broken down by enzymes and recycled. Cells can use this process to clear out the accumulation of disease-causing proteins, including those found in Huntington’s disease. In Huntington’s, however, autophagy is less effective than usual, leading to a toxic buildup of cellular waste.
In earlier work, co-author Juan Marugan, Ph.D., acting branch chief and group leader of the NCATS Chemical Genomics Center, and his NCATS colleagues tested thousands of compounds against the toxic Huntington’s protein in diseased cells. Scientists know that high levels of the protein in cells play a role in neurological defects in the disease. The team found that one compound, NCT-504, lowered the level of Huntington’s protein in the cells and improved the cells’ survival.
Marugan and his colleagues subsequently discovered that the compound worked by blocking PIP4Kγ activity. To better understand how this related to Huntington’s disease, the NCATS group, including co-authors Marc Ferrer, Ph.D., and Noel Southall, Ph.D., collaborated with co-author Lois Weisman, Ph.D., and her research team at the University of Michigan Life Sciences Institute in Ann Arbor.
The researchers knew that PIP4Kγ plays a role in cell signaling, a process by which cells send chemical messages to communicate information for biological activities. The team used NCT-504 to inhibit PIP4Kγ activity in various cells, including connective tissue cells from Huntington’s disease patients and human and mouse neurons from Huntington’s models. This inhibition of PIP4Kγ increased recycling activity and reduced the clusters of Huntington’s proteins in the cells.
The researchers also wanted to see what would happen if they genetically turned off PIP4Kγ activity. Co-author Juan Botas, Ph.D., Baylor College of Medicine in Houston, and his colleagues studied two different Huntington’s models, using fruit flies. The fruit fly model has been shown to mimic many of the effects of Huntington’s disease in humans. The neurons of Huntington’s fruit fly models are littered with toxic Huntington’s proteins. Silencing PIP4Kγ activity reduced the amount of Huntington’s disease protein that accumulated in fruit fly neurons, lessening the damage to the neurons and improving the flies’ ability to move.
“These results provide important insights into understanding the biology of a rare, devastating neurological disease,” said Marugan. “PIP4Kγ inhibitors could be useful for Huntington’s and other neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases, which also are marked by the accumulation of toxic proteins.”
Marugan and his colleagues plan to continue to evaluate molecules that are more efficient at blocking the enzyme. “We want to develop better molecules that could intervene in Huntington’s and potentially other diseases,” he said.
The research was supported by NCATS through its Intramural Research Program; National Institute of Neurological Disorders and Stroke grants R01-NS064015, R01-NS099340 and R01-NS097542 National Institute on Aging grant P30-AG053760; the American Heart Association; and the University of Michigan through its Protein Folding Diseases Initiative.


