Automated Retinal Screening Opens a Window into People’s Health
April 14, 2021
At a routine eye exam, NCATS grantee Kaushal Solanki, Ph.D., learned his abnormal retinal scan might be a sign of early-onset blindness. He needed to see an ophthalmologist fast, but it took four months for an appointment. Solanki was worried he could not wait that long. Having seen his father’s struggles with complications of diabetes, he knew all about the risks of diabetic retinopathy (DR), a disease that damages the blood vessels in the eye, as well as the shortage of qualified professionals to address the issue.

Kaushal Solanki, Ph.D., is the CEO and Founder of Eyenuk, Inc., where he leads the product development and execution of the company. (Eyenuk)
Today, it is difficult for eye care professionals to gauge the risk of DR and accurately monitor its progression. Catching DR early, when vision changes still are minor, can greatly reduce the progression to more severe disease and blindness. The medical challenge is how to visualize those early changes, called microaneurysm turnover, and use them as biomarkers for the disease. Current practices require expert examination of each scan and comparison to scans from a previous visit, a process that is cumbersome. As a result, it is nearly impossible to effectively track changes over time. What was needed, Solanki observed, was a technology that could improve the diagnosis and monitoring of retinal diseases and other conditions that can be reflected in the vasculature of the eye, such as cardiovascular disease.
Solanki, an artificial intelligence (AI) and machine learning scientist, thought his field could provide a solution. He remembered looking at his retinal scans for the first time and thinking that the blood vessels looked similar to roads and rivers visible in satellite imagery. Once he knew of the dire need for automated screening at point of care, the California-based researcher began building an AI-powered screening platform to support prevention and early detection. In 2010, Solanki founded Eyenuk, Inc., to make this vision a reality and make disease screening and monitoring more accessible and precise.
Novel AI Technology
Typically, when people with diabetes have their eyes examined for retinopathy, they must make an appointment with an eye specialist and then wait an average of six weeks for the next appointment. Solanki’s company created EyeArt® to automate this process with technology that can be used by any provider, including primary care providers and optometrists. EyeArt uses off-the-shelf fundus cameras to capture an image of the retina that can be uploaded to Eyenuk’s system in the cloud, where state-of-the-art AI technology analyzes the retinal images for minute changes that may be a sign of DR and other sight-threatening diseases. Within 60 seconds, the provider receives a report that can guide decisions about further treatment.
After developing EyeArt for on-the-spot diagnosis, it became clear to Solanki that clinicians needed a way to assess DR risk and progression in their patients over time. Eyenuk applied to NCATS in 2012 for small business funding to develop EyeMark™, which takes the EyeArt technology one step further by automatically analyzing and quantifying changes in the retina over time. By using the system to monitor DR progression, doctors can gauge the risk of blindness and evaluate treatment in patients. Solanki calls EyeMark’s technology “superpower AI” because it allows primary health care professionals to identify and follow tiny lesions on the retina.
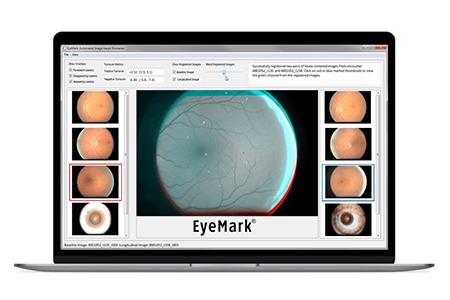
EyeMark™ takes fully automated diabetic retinopathy analysis one step beyond imaging, grading and reporting. (Eyenuk)
Through support from the NCATS Commercialization Readiness Pilot (CRP) program, Eyenuk is working to conduct clinical studies necessary for regulatory approval of EyeMark in the United States. Eyenuk also is collaborating with a partner to run trials of EyeMark with the U.K. National Health Service’s national screening program. EyeArt already is in use in clinics worldwide, including in the United States, Germany, Italy, Canada, Mexico, South Africa, India and in a national screening program run in Armenia.
Eyenuk is now harnessing the translational benefits of their AI algorithms to address other conditions. Currently, the company is developing screening tools for vision-threatening retinal diseases, such as age-related macular degeneration and primary open-angle glaucoma. Solanki is using the technology even to create detection tools for microvascular damage that could indicate other conditions, such as Alzheimer’s disease and cardiovascular disease.
“Eventually, we want to develop this platform that uses retinal imaging to provide information on what’s going on in the body,” Solanki said. “If the microvasculature is observed to have some issues in the eye, there is a hypothesis that this same microvasculature could be deteriorating in the heart or the kidneys or the foot. It’s very coordinated to what’s going on in the body.”
Small Business Innovation Research (SBIR) Funding with NCATS
“The NCATS SBIR program focuses on supporting transformative platform technologies, such as Eyenuk’s AI algorithms, that address various diseases,” stated Lili Portilla, director of NCATS’ small business programs. “We have discovered that small businesses like Eyenuk are essential to our mission of bringing more treatments to more people more quickly.”
Solanki credits NCATS’ small business funding and programs with helping Eyenuk cross the finish line toward accelerating their research to improve care and potentially apply to other disease areas.
“NIH grants provide the critical funding to kick-start and de-risk innovative projects and technologies that are very difficult to secure funding from venture capitalists or other private entities,” Solanki said. “We believe that we are on our way to realizing our vision of being able to screen every eye in the world.”
In addition to the SBIR and Small Business Technology Transfer programs, NIH and NCATS offer technical assistance to small businesses through several other programs. To learn more, visit the NCATS Other Small Business Programs page.


