DIAMOND Portal Offers Research Training for All
Competency Domains for the Clinical Research Professional
Sonstein, S.A., Seltzer, J., Li, R., Jones, C.T., Silva, H., Daemen, E. (2014, June). Moving from compliance to competency: A harmonized core competency framework for the clinical research professional (PDF - 259KB). Clinical Researcher. 28(3); 17-23.
Well-run clinical trials require well-trained staff. The people who work on clinical studies often have backgrounds in nursing, pharmacology, or other fields and then learn to run studies on the job. Researchers supported by NCATS’ Clinical and Translational Sciences Awards (CTSA) Program recognized a need for high-quality training materials and realized that academic medical centers already are developing them.
The DIAMOND Portal, launched in September 2018, brings these training materials together in one place. DIAMOND stands for Development, Implementation, and Assessment of Novel Training in Domain-based Competencies. The initiative began as a way to share materials developed at CTSA Program hubs, but now anyone can upload resources to the portal.
"Part of the vision of the CTSA Program is to be the catalyst to help move all clinical and translational research forward, so we are all charged with disseminating what we do," said Vicki Ellingrod, Pharm.D., co-principal investigator for DIAMOND at the Michigan Institute for Clinical and Health Research. DIAMOND was created in collaboration with the CTSA Program hubs at The Ohio State University, University of Rochester, and Tufts University.
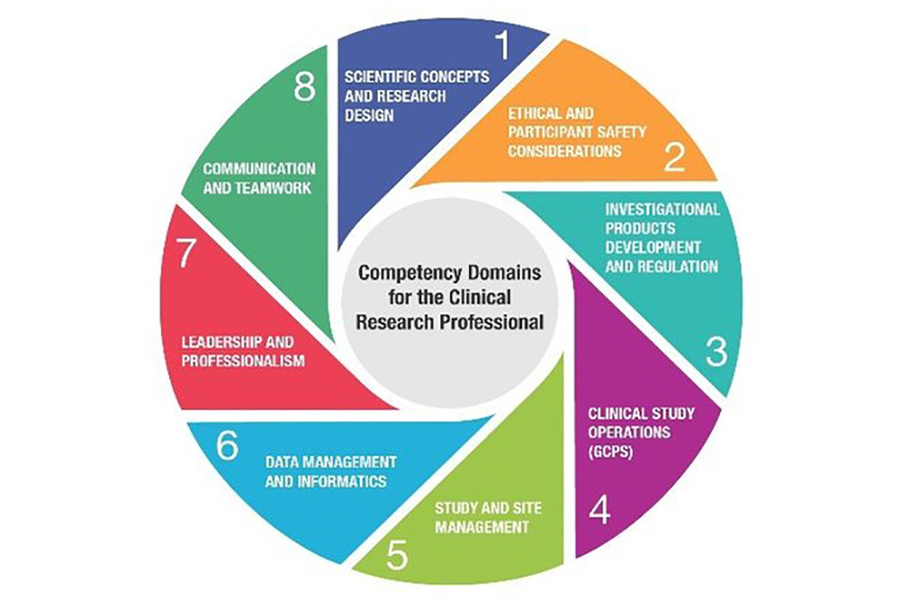
The portal includes training materials in eight different competency domains, ranging from the specifics of running trials to more general topics, such as leadership and professionalism. The portal also includes assessments that study teams can use to check that staff members are adequately trained. In addition, research professionals can boost their careers by maintaining an electronic portfolio of their training materials through DIAMOND. More than 17,000 people have accessed the portal so far, including many from outside the United States.


