New 3-D Models Offer Insights Into the Biology of a Rare, Devastating Neurological Disease
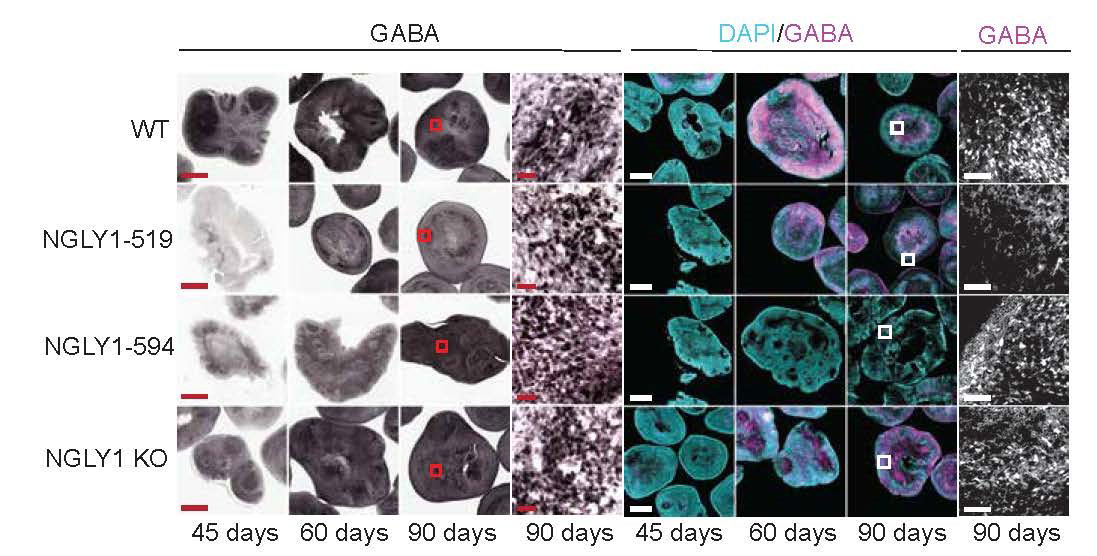
NCATS scientists and their colleagues created human cell–based, 3-D models (organoids) of the midbrain in NGLY1 deficiency, a rare neurological disease. Using the model, the researchers found changes in the development of neurons and reductions in GABA, an important chemical carrying messages in the brain. Here, the two middle rows represent NGLY1 deficiency organoids from patient cells. Scientists showed that GABA production is slower in NGLY1-deficient midbrain organoids than in the normal NGLY1 model, shown in the top row. (Abbott, Tambe, et al., Frontiers in Cell and Developmental Biology.)
July 28, 2023
NCATS scientists teamed with colleagues at Johns Hopkins University, The University of Alabama and Travere Therapeutics to create human cell–based models of the ultra-rare neurological disease NGLY1 deficiency. The models provide new insights into the disease’s development and biology. Ultimately, it may help researchers test drugs and treatments for the disease.
The new 3-D models, or organoids, are tiny versions of organs grown from stem cells in a laboratory dish. They can mimic a human organ’s structure and function. Scientists used the models to study NGLY1 deficiency’s effects on neurons in the midbrain, the part of the brain that is important in movement.
“Ultra-rare diseases often lack good animal models,” said NCATS translational scientist Wei Zheng, Ph.D., who led the work along with NCATS scientist and project manager Atena Farkhondeh, Ph.D. “Creating organoids from stem cells from people with NGLY1 deficiency offers a way to look at what goes wrong in the disease in a more realistic, human-like environment.”
The midbrain area controls the motor system. It is linked to movement disorders, such as Parkinson’s disease. The researchers hypothesized that a midbrain organoid model would reveal how NGLY1 deficiency may cause movement problems. Just like people with the disease, midbrain organoids with the disease lack NGLY1 protein.
“Patient-derived models offer the advantage of having the same genetic make-up as the patients,” Zheng said. “In mouse models of NGLY1 deficiency, the comparable gene is different than in humans.”
In Frontiers in Cell and Developmental Biology, the researchers, including co-first authors Joshua Abbott, Ph.D., and Mitali Tambe, Ph.D., described creating midbrain organoids from induced pluripotent stem cells. Such cells can be turned into any cell type in the body. The organoid cells came from two people, each with a different NGLY1 mutation — one with a severe form of the disease and one with a milder form. The researchers also created midbrain organoids from cells of people without the disease for comparison.
The researchers found changes in how neurons and several biological markers developed in the disease model compared to the normal model. There were lower levels of key enzymes, including one that helps make dopamine, and of another chemical, GABA, both of which carry signals from the brain to other parts of the body.
“These differences in dopamine- and GABA-producing neurons are consistent with what we see in NGLY1 deficiency patients,” Farkhondeh said. Dopamine plays roles in movement, memory and reward; GABA helps reduce stress and anxiety.
The results suggest that the organoids will be useful models for testing compounds and drugs for safety and effectiveness, both on their own and with animal studies.
“We’re trying to mimic the disease characteristics as closely as possible and correlate our findings with the actual disease,” Farkhondeh said. “By using patient cells to make organoid models, we’re creating good tools with which to potentially develop personalized therapies.”


