CTSA Program Support Kick-Starts Career and Emerging Research on Health Effects of Plastics
February 1, 2019
For newborns or children in the pediatric intensive care unit, plastic tubing is part of daily life. It delivers life-sustaining blood transfusions, liquid nutrition and air to breathe. But small amounts of the chemicals in the plastic of this tubing and other medical devices can leak into the patient’s bloodstream. The potential effects of these chemicals on the developing hearts of newborns and very young children are not well understood.
Early-stage investigator Nikki Posnack, Ph.D., an assistant professor at the Children’s National Heart Institute and Sheikh Zayed Institute for Pediatric Surgical Innovation, sought to close this knowledge gap. In 2016, she received pilot funding from the NCATS’ Clinical and Translational Science Awards (CTSA) Program hub at Children’s National Health System, kick-starting an emerging area of research — and her career.
“While plastics have revolutionized the medical field, we know chemicals in plastics leach into the body and may have unintended effects,” Posnack said. “The heart is sensitive to toxins, so we want to look at the effect of these plastics on the most sensitive patient population: kids who are recovering from heart surgery and already prone to cardiac complications.”
A Question Leads to a Career

Nikki Posnack, Ph.D., guides research to explore how chemicals used in medical devices and consumer products may alter heart function. (Children’s National Health System Photo)
Several years ago, Posnack was completing graduate research training on a team studying the heart’s electrical activity when she was approached by a toxicologist from the U.S. Food and Drug Administration. He wanted to know if the chemical DEHP, found in flexible medical devices such as plastic tubing, could affect heart function. Chemicals like DEHP are known to affect the body’s hormones and have been removed from many consumer products. But they remain in certain medical devices because they have properties needed for the devices and safer alternatives have not been extensively tested.
In the laboratory, Posnack began looking at the effects on heart cells of DEHP and other chemicals found in plastics. These early studies suggested that the chemicals could affect the cells’ electrical activity, especially over long periods of exposure.
What began as a collaboration would go on to become the focus of Posnack’s research as she started her own lab. After joining Children’s National in 2016, Posnack recognized that she would need new skills and methods to get to the bottom of this unanswered question.
Carving a Path as an Early-Stage Investigator
Posnack applied for CTSA Program pilot funding to adopt several critical techniques in her lab. One addressed how to determine the amount of chemicals from plastics that were leaking into a patient’s bloodstream. In collaboration with two nearby labs specializing in the technique of mass spectrometry, she honed the method using blood and urine samples from rats.
Mass spectrometry, which measures the mass of different molecules within a sample, enabled Posnack to see how much of the chemicals reached the rats’ hearts. This led to her first study in animal models. The results, published in the Nov. 6, 2017, issue of the American Journal of Physiology, showed that exposure to DEHP affected the nervous system’s ability to control heart rate following stress in adult rats. Moreover, the study was the first to show a link between chemicals called phthalates — one of which is DEHP — and heart toxicity in an animal model.
Posnack predicted that the developing heart of a newborn might be even more sensitive to the effects of chemicals from plastics. Using heart cells from newborn mice, grown in the lab, she examined the effects of another chemical, bisphenol A (BPA), which can also be found in plastic medical devices. BPA had substantial effects on the developing heart cells, leading to slower heart rates, irregular heart rhythms and skipped beats. That study, published in the May 9, 2018, issue of Scientific Reports, was the first to look at how BPA exposure affects heart cells that are still developing.
“People have known that BPA can have negative consequences on human health, but this research shows its effects may be much more extensive and could impact the hearts of young patients,” said Michael G. Kurilla, M.D., Ph.D., director of the NCATS Division of Clinical Innovation, which includes the CTSA Program. “Posnack is pioneering this emerging area of research that has significant public health implications.”
Toward a Better Translational Model
Although animal models provide evidence that a chemical might be toxic, there are differences — especially in the developing heart — that may limit the translation of findings from animal models to humans. To address this obstacle, Posnack wanted to develop a better model for young human heart cells. First, she had to learn how to work with induced pluripotent stem cells (iPSCs). These special cells, created in the lab from adult human skin or blood cells, can be coaxed into any kind of cell, including heart cells.
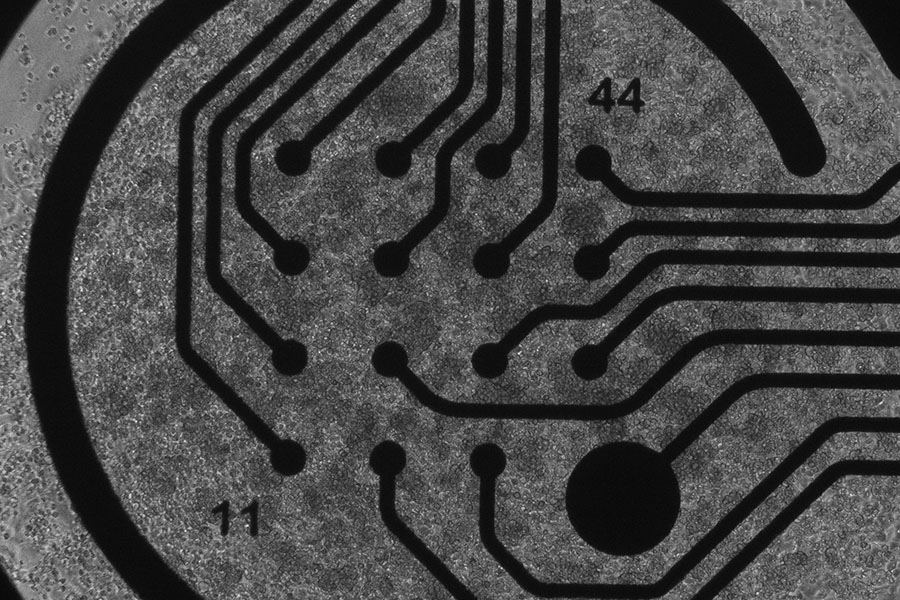
Human heart muscle cells, created from induced pluripotent stem cells, plated on a device to measure electrical activity. (Nikki Posnack Photo)
With the CTSA Program funding, Posnack sent a postdoctoral researcher to a collaborator’s lab to learn how to grow iPSCs and turn them into heart cells. She has since honed this technique in her lab and is developing a better model for looking at toxicity in the pediatric heart.
With this new skill and the ability to measure chemical exposure in patients, Posnack was awarded her first R01 grant, a five-year award from the National Heart, Lung, and Blood Institute, in 2018. The R01 is considered to mark the transition from an early-stage researcher to an independent investigator. Helping scientists navigate this career transition is a key CTSA Program goal.
“The CTSA Program funding provided the preliminary data that showed we could actually do the research to answer these critical questions and it wasn’t just a pipedream,” Posnack said.
Building the Evidence Base
Posnack is beginning to use her iPSC-derived heart model to screen chemicals from plastics currently in use in medical devices as well as alternatives that have recently come on the market. With independent funding and the techniques developed through the CTSA Program pilot project, she also has been collecting samples and vital sign data from pediatric patients who undergo medical procedures at Children’s National. This information will enable her to link high or low exposure levels with different outcomes, such as heart rate or blood pressure variability, which will help translate results from her iPSC model to humans.
“These chemicals are still used in so many medical devices,” Posnack said. “I think people are becoming aware of the negative effects of certain chemicals, and they will eventually be phased out. But it will require additional data to determine if these chemicals can be harmful to kids.”


