Biomedical Data Translator
We launched the Biomedical Data Translator program as a multiyear, iterative effort toward the eventual development of a comprehensive and relational “data translator” that combines multiple types of existing data sources.
Building a Data Translator
Contact
As a result of recent scientific advances, a tremendous amount of data is available from biomedical research and clinical interactions with patients, health records, clinical trials and adverse event reports that could be useful for understanding health and disease and for developing and identifying treatments for diseases. Ideally, these data would be mined collectively to provide insights into the relationship between molecular and cellular processes (the targets of rational drug design) and the signs and symptoms of diseases. Currently, these very rich yet different data sources are housed in various locations, often in forms that are not compatible or interoperable with each other. All of these factors limit the ability to get more treatments to all people more quickly.
To address these problems, we launched the Biomedical Data Translator program, called “Translator” for short. This multiyear, iterative effort will culminate in the development of a comprehensive, relational, N-dimensional Biomedical Data Translator that integrates multiple types of existing data sources, including objective signs and symptoms of disease, drug effects and intervening types of biological data relevant to understanding pathophysiology.
Biomedical Data Translator News
NCATS Team Creates Novel Computational Pipeline to Find New Ways to Treat Glioblastoma
March 10, 2025 - NCATS News
- Analytical Chemistry
- Biomedical Data Translator (Translator)
- Informatics (IFX)
- Our Impact on Drug Discovery and Development
- Our Impact on Rare Diseases
NCATS scientists tested a new method that could identify existing drugs effective against glioblastoma, a type of brain cancer that currently has no adequate treatments and a very low five-year survival rate.
Read ArticleTranslator for Biomedical Research Aims to Speed Up Patient Care
March 25, 2025 - Grantee/Partner News
- Biomedical Data Translator (Translator)
NCATS Team Creates Novel Computational Pipeline to Find New Ways to Treat Glioblastoma
March 10, 2025 - NCATS News
- Analytical Chemistry
- Biomedical Data Translator (Translator)
- Informatics (IFX)
- Our Impact on Drug Discovery and Development
- Our Impact on Rare Diseases
NCATS scientists tested a new method that could identify existing drugs effective against glioblastoma, a type of brain cancer that currently has no adequate treatments and a very low five-year survival rate.
AI Tool Helps Find Life-Saving Medicine for Rare Disease
February 5, 2025 - Grantee/Partner News
- Biomedical Data Translator (Translator)
- Our Impact on Rare Diseases
Translator Process
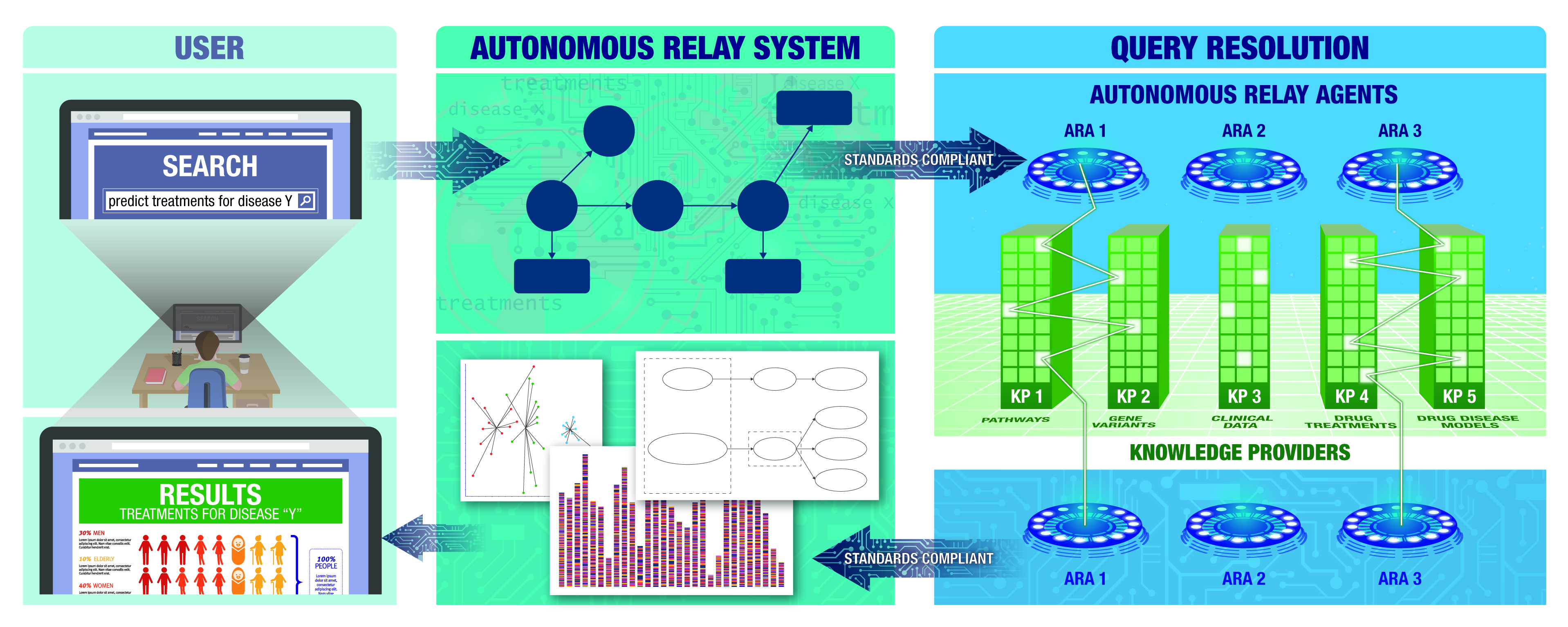
Translator Draft Architecture. A researcher will be able to use NCATS Biomedical Data Translator to request specific biomedical information. For example: “predict treatments for disease Y.” The query will be sent to Translator’s Autonomous Relay Agents (ARAs) to determine how best to answer the query. The ARA will break the query into smaller tasks that are transmitted to rich, specialty knowledge bases called Knowledge Providers (KPs). This process will be iterative, such that the ARAs and KPs can build on information from the others. Researchers will be able to explore this distilled knowledge and help them to develop new research hypotheses that lead to new scientific discoveries!
Funding Opportunities

There are currently no research opportunity announcements open for the Biomedical Data Translator program. Any further funding opportunities for this program in the future will appear here.
The most recent Translator funding opportunity (now closed) can be found here.
View questions and answers about the most recent Translator funding opportunity.
More About the Program

About Biomedical Data Translator
We provide more information about Translator and its objectives.

Translator Frequently Asked Questions
We have answers to frequently asked questions about the Biomedical Data Translator program.

Translator Projects
We are developing Translator to integrate the vast amounts of currently available medical research data and accelerate the development of new treatments.


