Stem Cell Translation Laboratory (SCTL)
The SCTL utilizes the latest technology and techniques in stem cell research to investigate innovative methodologies for accelerating the therapeutic development process.
About SCTL
Contact
The progress made in stem cell biology over the past decade has opened many new opportunities for basic and translational scientists worldwide. Induced pluripotent stem cells (iPSCs) are particularly useful because scientists can group them into many specialized cell types to use for research or regenerative medicine applications (e.g., cell and gene therapy).
About the Science
Scientists produce iPSCs by reprogramming adult cells (e.g., skin cells, blood cells) to an embryonic stem cell–like state, where they have the potential to become any cell type of the human body. iPSC-based therapies are ideal because they use a patient's own cells, preventing such potential complications as rejection by the immune system. In addition, scientists can use iPSCs to produce the large amounts of relevant cells (e.g., nerve cells, liver cells) needed for disease research, tissue engineering, toxicology and regenerative medicine. Major limitations that currently impede the application of iPSCs include the lack of highly reproducible and well-defined procedures required to generate, characterize and differentiate patient-specific iPSCs safely for preclinical and clinical use.
About the Lab
To establish and move stem cell technologies forward through a more centralized effort, NIH has launched the Stem Cell Translation Laboratory (SCTL) within NCATS. Initially part of the NIH Common Fund's Regenerative Medicine Program, the goal of the SCTL is to bring iPSC technology closer to clinical applications and drug development. Through the SCTL, NCATS provides researchers across various disciplines and organizations access to innovative protocols and resources to advance the translation of regenerative medicine applications. Through a multidisciplinary collaborative team approach, NCATS' SCTL scientists aim to:
- Establish quality control (QC) standards to define pluripotency and differentiated cell types
- Develop methods to assess heterogeneity and characterize iPSC-derived cells using multi-omics technologies
- Develop standardized methods to produce mature and functional cells meeting the QC standards above
- Discover, validate and disseminate small-molecule reagents to replace expensive recombinant proteins, xenogeneic material and undefined media components in cell differentiation protocols
SCTL News
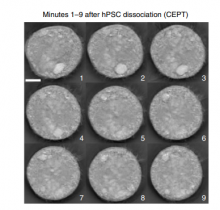
Better Method for Growing Stem Cells Could Speed Gene-Editing Therapies
April 7, 2023 - NCATS News
- Stem Cell Translation Laboratory (SCTL)
Scientists have published step-by-step instructions on how to use a four-part cocktail of drugs and compounds to grow stem cells from a single cell.
Read ArticleWorld’s First Patient Treated With Personalized CRISPR Gene Editing Therapy at Children’s Hospital of Philadelphia
May 15, 2025 - Grantee/Partner News
- Our Impact on Rare Diseases
- Stem Cell Translation Laboratory (SCTL)
Say Yes Until You Can Say No, Says NCATS’s Ryu
March 29, 2024 - Grantee/Partner News
- Stem Cell Translation Laboratory (SCTL)
Transforming Biomedical Research and Precision Medicine Through Induced Pluripotent Stem Cell Innovation
February 14, 2024 - Media Coverage
- Stem Cell Translation Laboratory (SCTL)
Virtual Tour of Stem Cell Translation Lab
This video (video length: 5:06) provides an overview of the SCTL lab and how NCATS is applying stem cells in translational science. Read Transcript.
Contact Us
If you are an NIH Investigator, you can submit your proposal to the Functional Genomics Lab.
Connect with Carlos Tristan, Ph.D., to get started.
Related Research
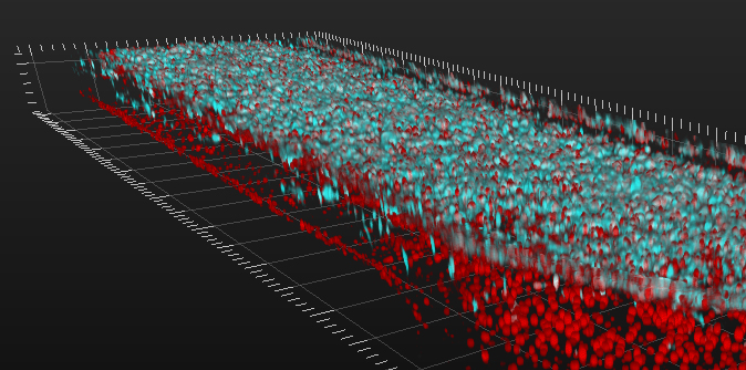
3-D Tissue Bioprinting
Our scientists are creating and using 3-D printing techniques to make tissue models that closely resemble the complex structure and organization of our cells.
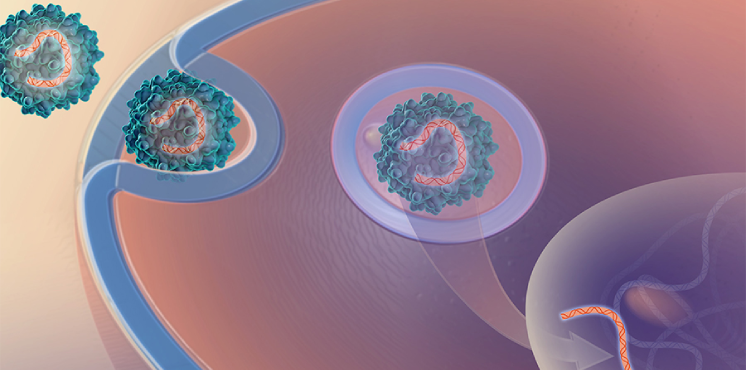
Platform Vector Gene Therapy (PaVe-GT)
Our pilot project is testing the feasibility of increasing the efficiency of gene therapy clinical trials by using the same gene delivery system and manufacturing methods for multiple gene therapies.

Assay Development and Screening Technology (ADST)
Our scientists explore and develop new ways to quickly test and screen potential drug candidates, often in collaboration with disease foundations.


