November 12, 2024: Easing the Burden of Rare Diseases Through Translational Science
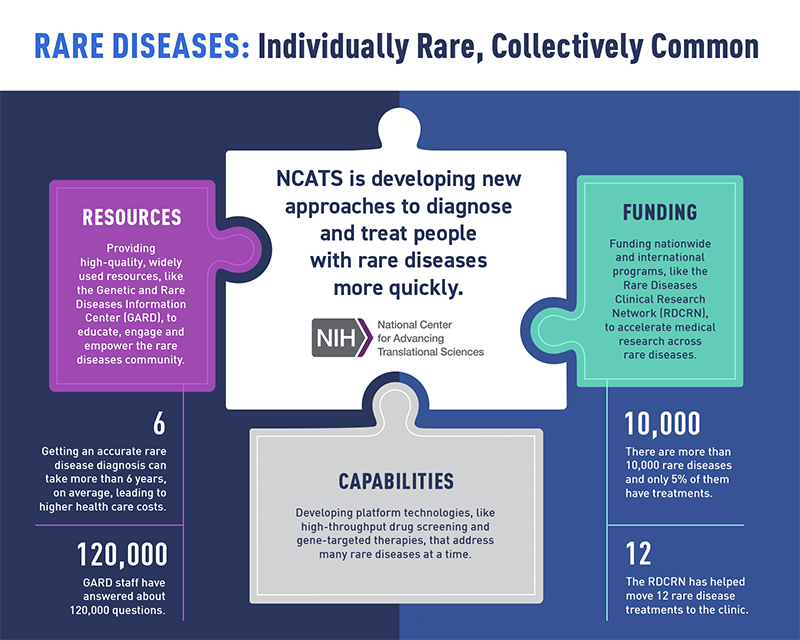
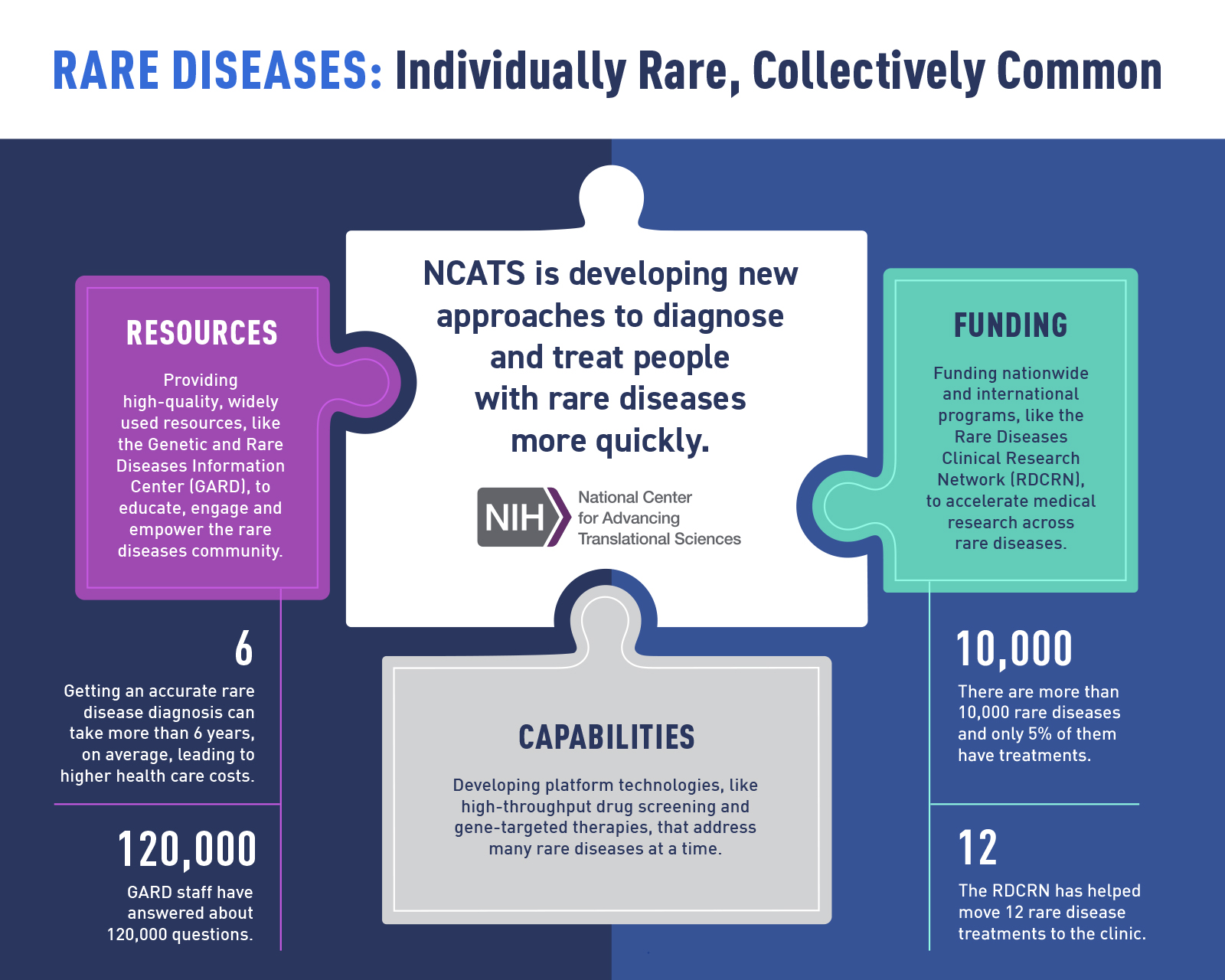
NCATS is developing new approaches to diagnose and treat people with rare diseases more quickly through resources, capabilities, and funding. (NCATS)
The term “rare disease” can be a misnomer. While there are more than 10,000 diseases that are individually rare, they collectively affect millions of people worldwide.
The burden for people with a rare disease is significant. Most rare diseases start in childhood. However, only about 5% of all rare diseases have U.S. Food and Drug Administration-approved treatments. Countless doctor visits, tests, referrals, and — even worse — wrong diagnoses take a large toll on patients and their families. This journey also leaves them with annual health care costs three to five times greater than those for a person without a rare disease.
Advancing the development of and access to more treatments for diseases with unmet needs is a strategic priority for us, and finding solutions for rare diseases is a perfect fit for translational science. This field addresses the scientific and operational roadblocks that slow progress. It also makes the way research is done faster, more efficient, and more impactful.
By using translational science approaches, we’ve made a lot of progress in rare diseases research — from moving dozens of promising treatments from the lab to the clinic to providing widely used community resources. But as research capabilities and knowledge keep growing, so too does the to-do list. Key areas we’re actively working on include:
- Understanding rare disease biology and therapeutic targets, especially common features
- Building platform approaches that advance drug discovery and development efforts for many diseases
- De-risking promising new treatments so they’re more attractive for commercial development
- Leveraging new technologies, such as AI/machine learning and whole-genome sequencing, to improve diagnostic approaches
- Modernizing clinical trial approaches for gene therapies and other newer treatment modalities
- Bringing partners together to ensure health solutions reach the people who need them the most
In my new Director’s Message series, I will cover major pain points, progress, and priorities in rare diseases research across the translational pipeline. I hope you find the messages informative and that they spark new conversations on ways we can work together to address the public health challenge of rare diseases.
Your partner in science and health,
Joni L. Rutter, Ph.D.
Director
National Center for Advancing Translational Sciences