OpenData Portal
The OpenData Portal is a free online platform that offers real-time information about how individual SARS-CoV-2 variants may respond to known therapeutics.
About the OpenData Portal
One potential strategy against SARS-CoV-2, the novel coronavirus behind the disease COVID-19 and a worldwide pandemic, is to use old drugs in new ways. This approach is called drug repurposing and can cut the time it takes to develop U.S. Food and Drug Administration–approved drugs from as long as 10 to 15 years to just 1 to 2 years. To further reduce this timeline, we created a new resource for scientists, the OpenData Portal (ODP), to openly and quickly share COVID-19-related drug repurposing data and experiments for all approved drugs.
How the OpenData Portal Works
The ODP includes SARS-CoV-2 screening data on more than 10,000 drugs and compounds, including the nearly 3,000 approved drugs in the NCATS Pharmaceutical Collection. The resource includes information on assays (tests), protocols for using the assays, drug targets, mechanisms of drug action and screening assay data. These data, which include positive and negative results, can be viewed, sorted, searched and exported from the portal website. Screening data are uploaded to the website as they become available. All data on the site come from NCATS-validated SARS-CoV-2 assays. The ODP amasses all industry tests of new and existing COVID-19 therapeutics and catalogs all research sources, with more than 10,000 sources collected to date.
The scientific community can use the data for a variety of drug repurposing activities, allowing them to formulate and test hypotheses, prioritize research opportunities, and speed the search for effective therapies against the virus and the disease it causes.
Related Research

Our Impact on Big Data
Our teams find ways to transform massive amounts of data into health solutions faster than ever.
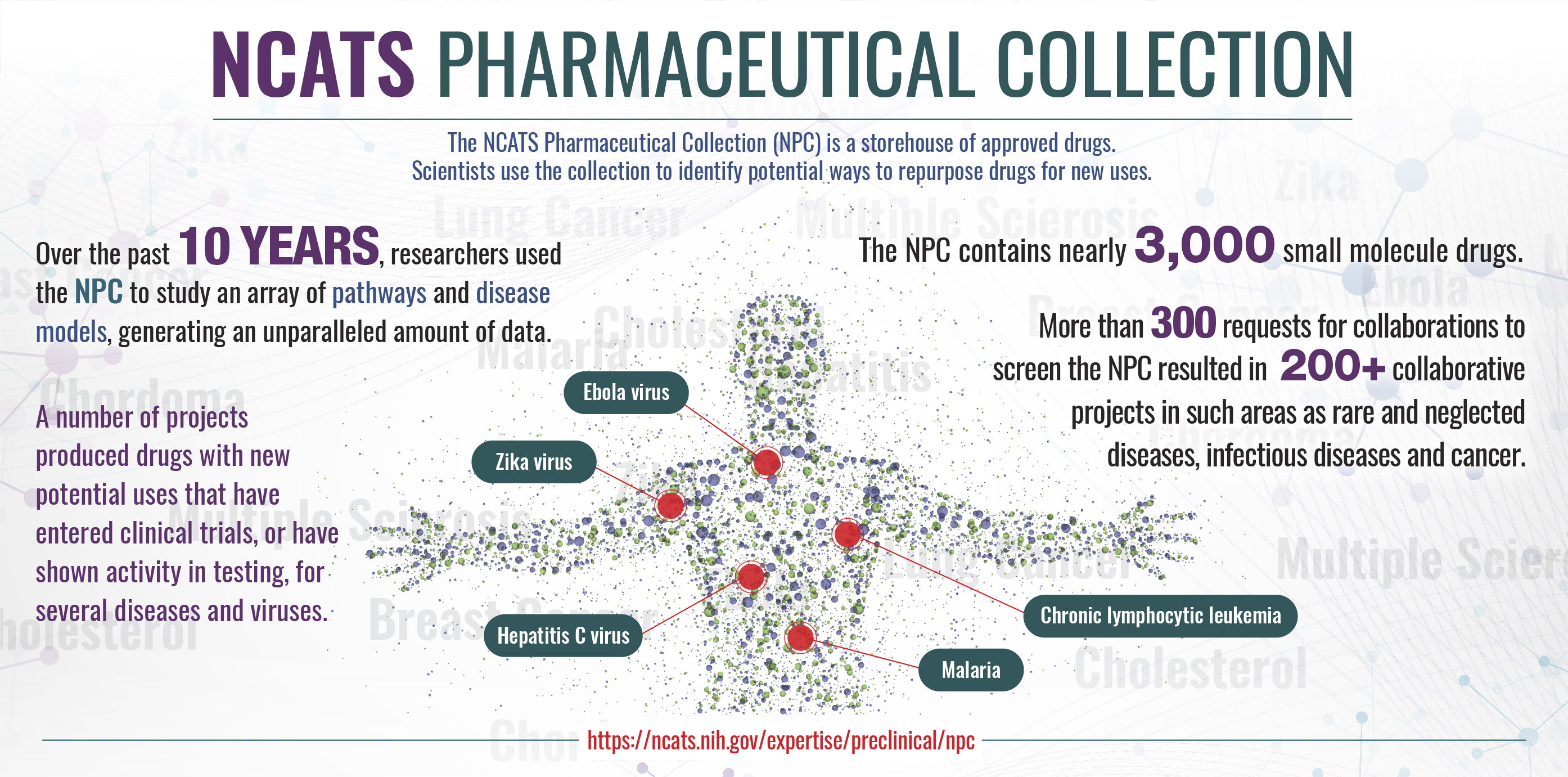
NCATS Pharmaceutical Collection
The National Pharmaceutical Collection is a complete, publicly available collection of approved molecular parts for high-throughput screening.
National Clinical Cohort Collaborative (N3C)
N3C is a national resource of real-world data that researchers are using to speed medical research.


