NIH HEAL Initiative Funding & Collaboration Opportunities Led by NCATS
As part of the National Institutes of Health (NIH) Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®, we support and facilitate a variety of funding and collaboration opportunities designed to address the opioid crisis, including pain management.
NIH HEAL Initiative Funding & Collaboration Opportunities Led by NCATS
Current Funding Opportunities
See our funding page for open HEAL opportunities.
Translational Research Collaboration Opportunities
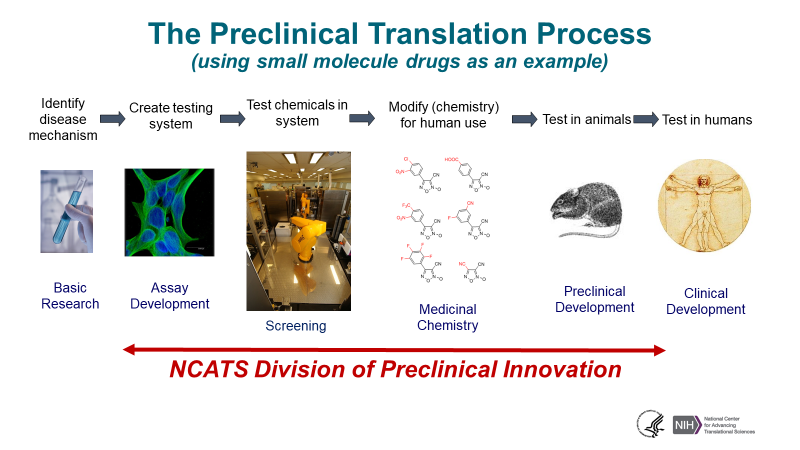
How do I:
- Translate my animal-based research discovery into a human-based model for continued testing and development?
- Advance my research on a potential therapeutic from plated cells into more complex, human cell–based models?
Develop a small molecule “probe” compound that can test the therapeutic hypothesis in cell-based or animal model systems? Are these questions that you are wrestling with on your therapeutic development project? NCATS welcomes your NIH HEAL Initiative–related proposals to develop novel probes and human cell–based models in team-based research collaborations with NCATS’ intramural scientists in the Division of Preclinical Innovation.
- As research collaborators, you bring a wealth of background knowledge and a starting point for a particular translational project.
- NCATS scientists provide expertise and resources to transform those starting points into therapeutically useful tools, platforms or probe molecules.
- Approved projects result in formation of joint project teams that will work together to design and follow milestone-driven project plans to achieve agreed-upon deliverables.
Learn more about collaborating with NCATS scientists and how to submit a proposal.
The NIH HEAL Pain Management Effectiveness Research Network (NIH HEAL Pain Management ERN)
Using NCATS’ Clinical and Translational Science Awards Trial Innovation Network, the NIH HEAL Pain Management ERN will support studies to compare the effectiveness of existing pain treatments and novel approaches to prevent and manage pain while reducing the risk of addiction. The goal is to provide clinicians with information about the effectiveness of treatments or management strategies that reduce opioid use and pain associated with many types of diseases or conditions. Learn more about how the NIH HEAL Pain Management ERN will use the Trial Innovation Network for these studies.



