Scientists Discover Potential Treatment Approaches for Polycystic Kidney Disease
April 4, 2024
Innovative disease modeling and gene editing techniques begin to answer long-standing questions
Researchers have shown that dangerous cysts, which form over time in polycystic kidney disease (PKD), can be prevented by a single normal copy of a defective gene. This means the potential exists that scientists could one day tailor a gene therapy to treat the disease. They also discovered that a type of drug, known as a glycoside, can sidestep the effects of the defective gene in PKD. The discoveries could set the stage for new therapeutic approaches to treating PKD, which affects millions worldwide. The study, partially funded by the National Institutes of Health (NIH), is published in Cell Stem Cell.
Scientists used gene editing and 3-D human cell models known as organoids to study the genetics of PKD, which is a life-threatening, inherited kidney disorder in which a gene defect causes microscopic tubes in the kidneys to expand like water balloons, forming cysts over decades. The cysts can crowd out healthy tissue, leading to kidney function problems and kidney failure. Most people with PKD are born with one healthy gene copy and one defective gene copy in their cells.
“Human PKD has been so difficult to study because cysts take years and decades to form,” said senior study author Benjamin Freedman, Ph.D., at the University of Washington, Seattle. “This new platform finally gives us a model to study the genetics of the disease and hopefully start to provide answers to the millions affected by this disease.”
To better understand the genetic reasons cysts form in PKD, Freedman and his colleagues sought to determine if 3-D human mini-kidney organoids with one normal gene copy and one defective copy would form cysts. They grew organoids, which can mimic features of an organ’s structure and function, from induced pluripotent stem cells, which can become any kind of cell in the body.
To generate organoids containing clinically relevant mutations, the researchers used a gene editing technique called base editing to create mutations in certain locations on the PKD1 and PKD2 genes in human stem cells. They focused on four types of mutations in these genes that are known to cause PKD by disrupting the production of polycystin protein. Disruptions in two types of the protein – polycystin-1 and polycystin-2 – are associated with the most severe forms of PKD.
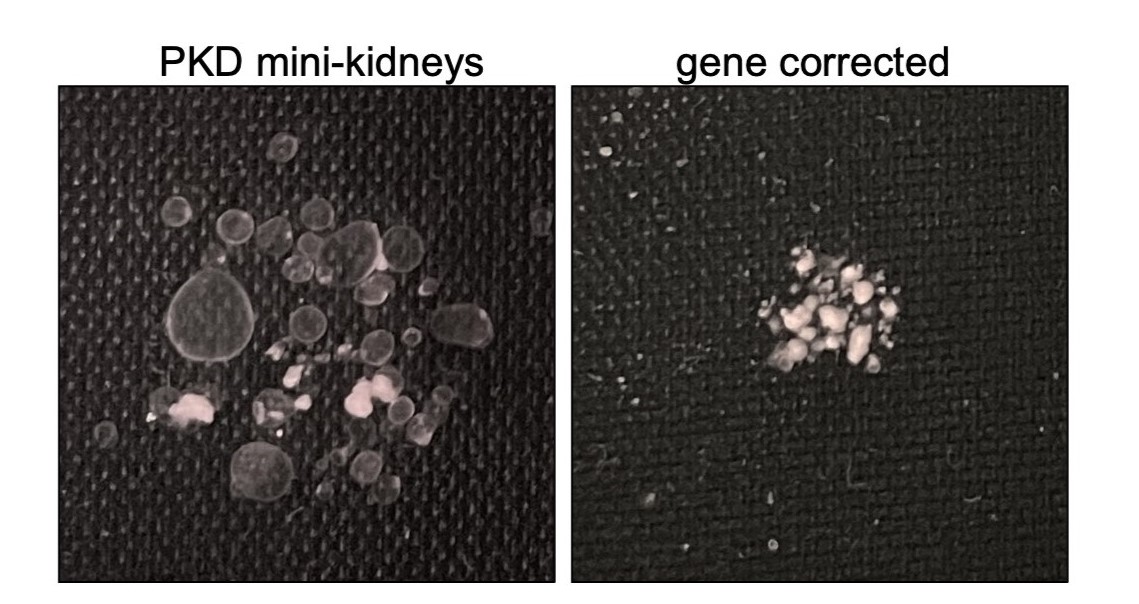
They then compared cells with two gene copy mutations in organoids to cells with only one gene copy mutation. In some cases, they also used gene editing to correct mutations in one of the two gene copies to see how this affected cyst formation. They found organoids with two defective gene copies always produced cysts and those that carried one good gene copy and one bad copy did not form cysts.
“We didn’t know if having a gene mutation in only one gene copy is enough to cause PKD, or if a second factor, such as another mutation or acute kidney injury was necessary,” Freedman said. “It’s unclear what such a trigger would look like, and until now, we haven’t had a good experimental model for human PKD.”
According to Freedman, the cells with one healthy gene copy make only half the normal amount of polycystin-1 or polycystin-2, but that was sufficient to prevent cysts from developing. He added that the results suggest the need for a second trigger and that preventing that second hit might be able to prevent the disease.
The organoid models also provided the first opportunity to study the effectiveness of a class of drugs known as eukaryotic ribosomal selective glycosides on PKD cyst formation.
“These compounds will only work on single base pair mutations, which are commonly seen in PKD patients,” explained Freedman. “They wouldn’t be expected to work on any mouse models and didn’t work in our previous organoid models of PKD. We needed to create that type of mutation in an experimental model to test the drugs.”
Freedman’s team found that the drugs could restore the ability of genes to make polycystin, increasing the levels of polycystin-1 to 50% and preventing cysts from forming. Even after cysts had formed, adding the drugs slowed their growth.
Freedman suggested that a next step would be to test existing glycoside drugs in patients. Researchers also could explore the use of gene therapy as a treatment for PKD.
The research was supported by NIH’s National Center for Advancing Translational Sciences, National Institute of Diabetes and Digestive and Kidney Diseases, and National Institute of General Medical Sciences through awards R01DK117914, UH3TR002158, UH3TR003288, U01DK127553, U01AI176460, U2CTR004867, UC2DK126006, P30DK089507, R21DK128638, and R35GM142902; an Eloxx Pharmaceuticals Award; the Lara Nowak-Macklin Research Fund; and a Washington Research Foundation fellowship.
About the National Center for Advancing Translational Sciences (NCATS): NCATS conducts and supports research on the science and operation of translation — the process by which interventions to improve health are developed and implemented — to allow more treatments to get to more patients more quickly. For more information about how NCATS helps shorten the journey from scientific observation to clinical intervention, visit https://ncats.nih.gov.
About the National Institutes of Health (NIH): NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical and translational medical research, and is investigating the causes, treatments and cures for both common and rare diseases. For more information about NIH and its programs, visit https://www.nih.gov.
NIH…Turning Discovery Into Health®