Sugar Compound’s Key Role in SARS-CoV-2 Infection May Lead to New COVID-19 Therapies
March 11, 2024
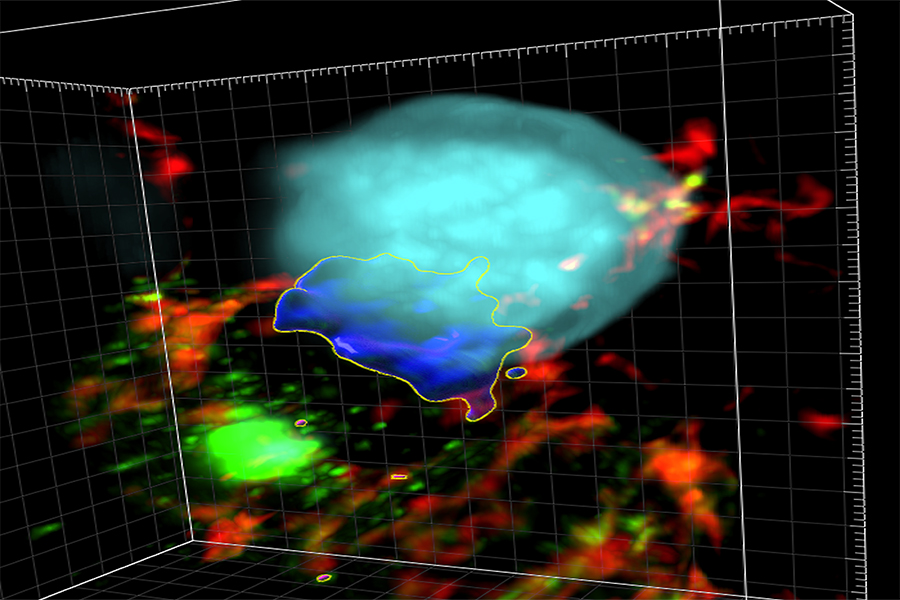
NIH scientists and their colleagues have found new clues to how SARS-CoV-2, the virus that causes COVID-19, can infect cells. They found that heparan sulfate (HS), a sugar compound that sits on the surface of a cell, serves as a key accomplice in creating “super-clusters” of the ACE2 protein, the entry point for the virus. SARS-CoV-2 binds to ACE2 when it infects a cell. Super-clusters are key to the process.
The findings, detailed in Nature Communications, provide new insights into how SARS-CoV-2 exploits a cell’s machinery and could open new ways to develop therapies to block viral infection.
The researchers, led by NCATS translational scientists Wei Zheng, Ph.D., and Qi Zhang, Ph.D., and Yihong Ye, Ph.D., at the National Institute of Diabetes and Digestive and Kidney Diseases, further showed that HS drives the formation of syncytia (many cells fused together). This helps the virus spread.
“This was an unexpected finding,” said Zheng. “These results could mean a new way of looking at different points in the virus infection, and new vulnerabilities to examine in how the virus interacts with the cell.”
The discovery sheds light on an underexplored part of viral infection — how the host cell helps the virus enter it and the virus’s ability to reproduce, said Zhang.
“By understanding this mechanism, scientists can target the interaction between heparan sulfate and the virus,” he explained. “This can potentially reduce the virus’s ability to infect host cells.”
In 2020, the group first reported in Cell Discovery that HS was involved in SARS-CoV-2 infection. The researchers screened an NCATS collection of U.S. Food and Drug Administration–approved drugs and found that two drug classes could block HS activity and prevent infection. They then studied one of the drugs, mitoxantrone, to learn more about how it could block infection.
In their recent study, the scientists wanted to learn how the fusing of cells boosted SARS-CoV-2 infection and spread. They focused on getting more evidence about HS’s role in SARS-CoV-2 infection. The researchers tested the effects of a pair of repurposed drugs they had previously identified for their effect on HS. The tests were done on organoids, which are miniature versions of organs grown from stem cells in a laboratory dish. They found that by blocking HS activity, the drugs blocked the formation of super-clusters and syncytia. This stopped viral infection in cells in human lung organoids and in a mouse model.
The scientists also learned more about the role and mechanism of cell fusion in SARS-CoV-2 infection.
“We know that cell fusion requires virus-infected cells to connect the spike protein on the virus to the ACE2 protein receptor,” Ye said. “HS brings infected cells and ACE2-positve cells together to cause the fusion. But the viral particle is so small that when it fuses with the cell, it doesn’t bring many virus spike molecules to the cell surface. We found the ACE2 clusters help to reconcentrate spike protein and promote cell fusion.”
A better understanding of the process behind SARS-CoV-2 infection, said Zheng, “allows new opportunities for developing innovative treatments that target the virus–host interaction.”
The researchers plan to study different versions of mitoxantrone and other similar compounds to see if they can make them more effective against SARS-CoV-2 infection.