PaVe-GT: Collaborative NIH Effort Aimed at Creating a Gene Therapy Playbook, Making Rare Disease Treatments More Accessible
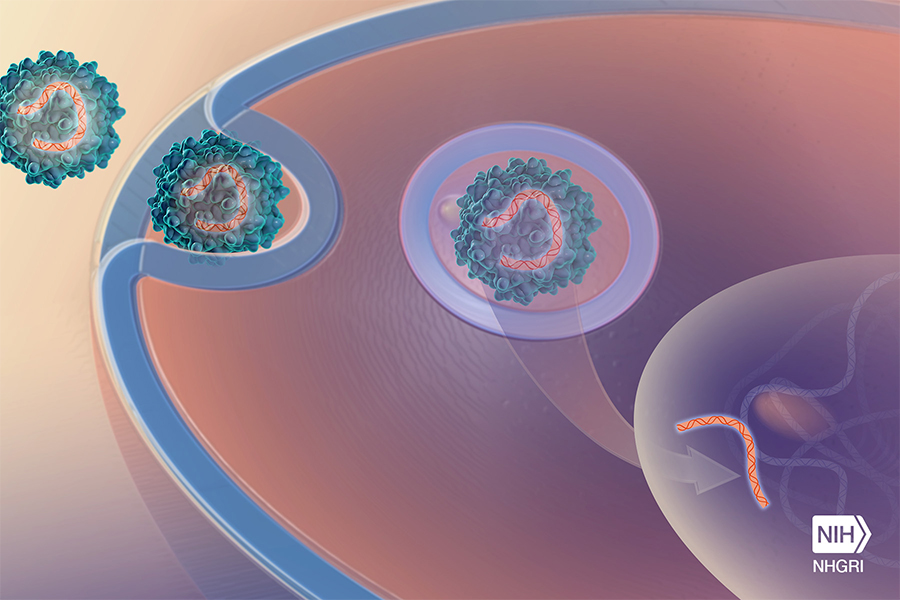
PaVe-GT is aimed at streamlining the development of gene therapies for rare diseases. Here, an adeno-associated virus carries a gene into a cell. (Darryl Leja, NHGRI)
November 30, 2020
New project will test whether using the same gene therapy delivery system and manufacturing methods can speed rare disease clinical trials
A new NIH initiative spearheaded by NCATS aims to make gene therapy development and clinical testing more streamlined and less expensive — and potentially more accessible to millions of people with rare diseases.
Only about 5% of the approximately 7,000 rare diseases have a treatment approved by the U.S. Food and Drug Administration (FDA). Most of these disorders are caused by a defect in a single gene, meaning many potentially can be treated by gene therapy. Gene therapy entails replacing a malfunctioning gene with a working version.
New technologies — including the use of a modified virus to deliver genes to cells that need properly functioning genes — are making gene therapy an increasingly attractive treatment option for individuals with rare diseases. Yet, thousands of disorders are so rare that companies may be reluctant or unable to invest the years of research and millions of dollars necessary to develop, test and bring a gene therapy for one disease to market.
NCATS, in conjunction with NIH’s National Human Genome Research Institute (NHGRI), National Institute of Neurological Disorders and Stroke (NINDS), and Eunice Kennedy Shriver National Institute of Child Health and Human Development, recently launched the Platform Vector Gene Therapy (PaVe-GT) initiative to test whether it is feasible to use the same gene delivery system and manufacturing methods for multiple rare diseases in gene therapy clinical trials. Using a common delivery system and manufacturing method makes gene therapy for rare diseases more accessible because the costs for therapeutic development and testing are shared.
“We’re pretty good at preventing, treating and even curing single-gene diseases in animal models, but very few candidate gene therapies get to clinical trials, often because of the cost and the complexity of developing a clinical trial for a single disease,” said P.J. Brooks, Ph.D., a program officer in the NCATS Office of Rare Diseases Research and one of the leaders of the initiative. “Most rare diseases are not commercially viable for companies to invest in, and patients have to find other resources.”
For scientists to make a dent in treating thousands of rare diseases, Brooks said, “We have to take advantage of relative commonalities among diseases and adopt a many-diseases-at-a-time approach.”
In a recent publication in Human Gene Therapy, Brooks and PaVe-GT colleagues described the make-up of the pilot project. PaVe-GT researchers will use a common gene delivery vehicle, adeno-associated virus (AAV), for gene therapy to treat four rare genetic diseases. They plan to use the same AAV delivery system to carry a therapeutic gene to the right place in the body but switch the cargo — the gene — for each disease.
Each disease currently is being studied at the NIH Clinical Center. They include two inherited muscle weakness/neuromuscular junction disorders (Dok7 deficiency and Collagen Q deficiency) and two inherited metabolic diseases (propionic acidemia and isolated methylmalonic acidemia). The neuromuscular junction is the site where a nerve fiber and a muscle cell chemically communicate. “While we’re making progress against these disorders that we’ve been studying for years, we also hope we can improve the efficiency of the gene therapy clinical trial process and develop a blueprint for other similar rare disease gene therapy projects,” said NHGRI geneticist and study leader Charles Venditti, M.D., Ph.D.
PaVe-GT researchers also suggest that many preclinical studies, such as those that examine the toxicity and distribution of an investigational therapy in the body, could prove useful for multiple disorders, further streamlining gene therapy development.
Transparency matters
To enable others to understand and learn from the scientific and procedural progress of PaVe-GT, scientists will share their data publicly. They will work closely with FDA officials to chart the progress on preclinical studies, including the safety, toxicity and distribution of an investigational therapy in the body. PaVe-GT researchers and their FDA colleagues will discuss such project aspects as gene manufacturing, gene delivery and clinical trial design. These conversations and subsequent FDA feedback — normally considered proprietary by companies — will be available to the public on the PaVe-GT website as well.
“Some companies are already using similar approaches to study groups of diseases, but certainly not in such a systematic and transparent way,” said Carsten Bönnemann, M.D., an NINDS pediatric neurologist and neurogeneticist who leads two of the studies. “Our goal is to show the feasibility of and the potential roadblocks — as well as opportunities — in an approach to treating more than one disorder at a time. People can track the entire gene therapy development process, from the preclinical studies in the lab to a clinical trial.”
“Most smaller companies would never have access to these types of studies and documentation that go into developing a gene therapy,” said Venditti. “We’re hoping this information is helpful to rare disease organizations as well, which in some cases are attempting to study a disease on their own. Perhaps our results will encourage these organizations and academic investigators to go after diseases that really need new treatments.”
One such disease foundation is Cure Raghav, which is led by Sanath Ramesh, a software engineer in Seattle. His son, Raghav, has an ultra-rare, fatal disease. After working with various clinical and scientific experts and exploring treatment options for his son, Ramesh turned his attention to gene therapy as a long-term solution. “I quickly realized the financial and logistical hurdles, as well as the lack of time and resources, that make this so difficult,” he said. PaVe-GT, he noted, “is an important step to meeting the needs of the rare disease community.”
“PaVe-GT is akin to creating a generic gene therapy,” said Donald Lo, Ph.D., an NCATS translational scientist and senior author on the Human Gene Therapy paper. “Rare disease groups and scientists will be able to use this playbook and adapt it to their needs, avoiding having to raise the many millions of dollars it currently takes to access proprietary gene therapy technologies. If it works, it potentially will be transformational for the field.”


