Lung Tissue Chips Could Help Predict Future Infectious Influenza Disease Variants
November 17, 2021
Using a tiny tissue chip that models the human lung airway, NCATS-funded translational scientists have replicated in a laboratory how influenza viruses evolve as they travel from one person to another. Their innovative tool could sharpen predictions about the rise of dangerous new virus strains, slow the rise of drug-resistant variants and lead to more effective vaccines and treatments.
Viruses such as influenza and SARS-CoV-2 mutate rapidly, fueling drug-resistant variants and frustrating vaccine development. The ever-evolving flu virus presents a particular challenge for scientists trying to predict the predominant strains in an upcoming flu season and produce effective vaccines.
In a study published in Microbiology Spectrum, researchers at the Wyss Institute for Biologically Inspired Engineering at Harvard University detailed how a lung airway-on-a-chip, or lung tissue chip, allowed them to accurately mimic the real-world evolutionary paths flu viruses take as they’re passed among people treated with antiviral drugs.
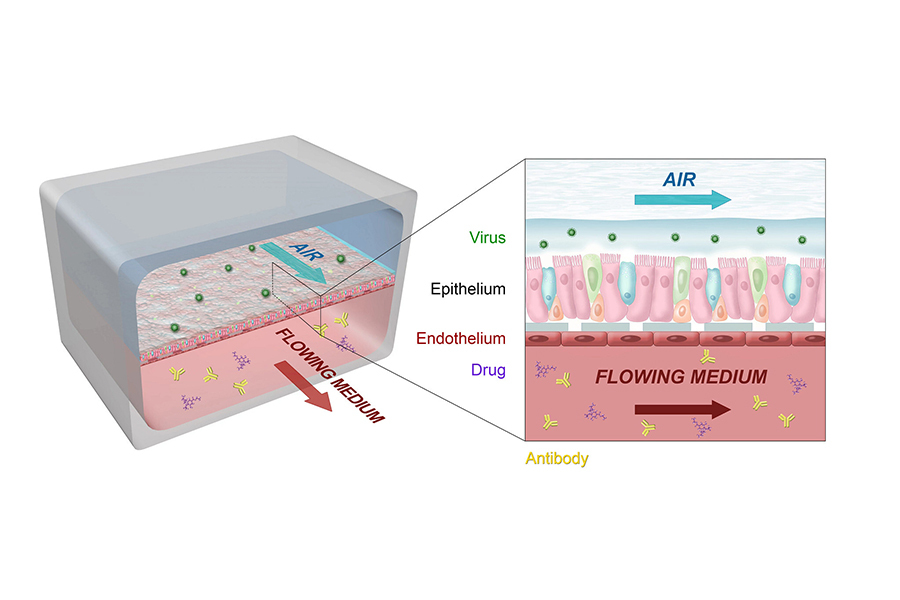
A cross-disciplinary team of engineers, biologists, virologists, immunologists and other scientific experts worked together to develop and customize the technology behind the lung airway tissue chip, a tiny, bioengineered, three-dimensional model of the human lung airway. The chip features two channels — an air channel and a blood channel — separated by human lung tissue on a porous membrane. Each channel is about the width of the lead in a pencil. As in a real human lung, lung airway epithelial cells face the air channel, while pulmonary microvascular endothelial cells face the blood channel.
The researchers infected the lung cells with airborne flu virus via the chip’s air channel, then treated the cells with antiviral drugs via the blood channel. To mimic human-to-human transmission, they then passed the virus from one tissue chip to another tissue chip, repeating the infection-and-treatment process.
Using an influenza A (H1N1) strain that is sensitive to two antiviral drugs — amantadine and oseltamivir — the researchers measured how many passages from chip to chip that it took for the virus to develop mutations that made it resistant to drug treatment. They also tracked the mutations that arose as the virus moved from one chip to another.
Within eight rounds of chip-to-chip passage, amantadine exposure led to a pool of variants that left the drug able to inhibit less than 10% of viral replication. Oseltamivir resistance took longer to develop — 25 passages from chip to chip. In contrast, nafamostat, a protease inhibitor drug that alters cells’ reactions to viruses, rather than attacking viruses directly, induced no viral resistance, even after 30 passes from chip to chip.
A closer look at the variants’ mutations highlighted the lung airway chip’s real-world predictive potential. A key mutation conferring amantadine resistance in the lung airway tissue chips is frequently detected in humans who have contracted amantadine-resistant flu viruses. And a key mutation in the tissue chip–induced oseltamivir-resistant flu strain is present in real-world, clinical cases of oseltamivir resistance. The lung tissue chip model also revealed potential mutations not yet seen clinically.
“We were able to show that some antiviral drugs are better [than others] at preventing the emergence of drug resistance,” explained Donald Ingber, M.D., Ph.D., founding director of the Wyss Institute at Harvard University, who led the work. “That knowledge could help clinicians prioritize which drugs they should give first, and even identify effective antiviral combination therapies.”
The lung airway-on-a-chip’s predictive potential could extend far beyond influenza viruses. “Drug resistance could be studied with any sort of pathogen that evolves quickly,” Ingber noted. “You could also use these chips to develop diagnostics that detect infections sooner and widen a patient’s therapeutic window.”
The lung airway-on-a-chip also may be an integral piece in solving larger disease-specific puzzles. Harvard researchers are developing organ chips that can be linked together to model diseases and immune system responses in conditions ranging from chronic obstructive pulmonary disease to cystic fibrosis. For example, linking a lung airway-on-a-chip to an immune system lymphoid follicle tissue chip could help researchers predict the effectiveness of an immunization against infection by airborne pathogens. Research using such tissue chip combinations is backed by NCATS through the Biomedical Advanced Research and Development Authority (BARDA) ImmuneChip+ program, which supports the creation of tissue chips that replicate the interaction of human organ tissues and the immune system.
“Tissue chips are a powerful translational science tool that can speed the transformation of basic drug discoveries into effective treatments,” said Danilo Tagle, Ph.D., director of NCATS’ Office of Special Initiatives. “The Wyss Institute’s approach could offer a cutting-edge 3-D model to predict viral evolution, as well as human responses to therapies. With tissue chips, we could prevent many drug failures, create safer drug profiles and predict which drugs will be more effective in different populations.”