3-D Tissue Bioprinting Operational Model
Our projects are partnerships with members of the broad biomedical community, who provide expertise on disease physiology, access to patient-derived induced pluripotent stem cells and primary cells, and access to disease animal models. In turn, we provide expertise in tissue engineering, assay development and drug screening.
About Our 3-D Tissue Bioprinting Operational Model
We work collaboratively with NIH intramural and extramural investigators and clinicians, regulatory agencies, biopharma industry groups and technology providers to advance, demonstrate and disseminate the development, application and use of 3-D tissue models for advancing therapeutics discovery and development.
History of the Program
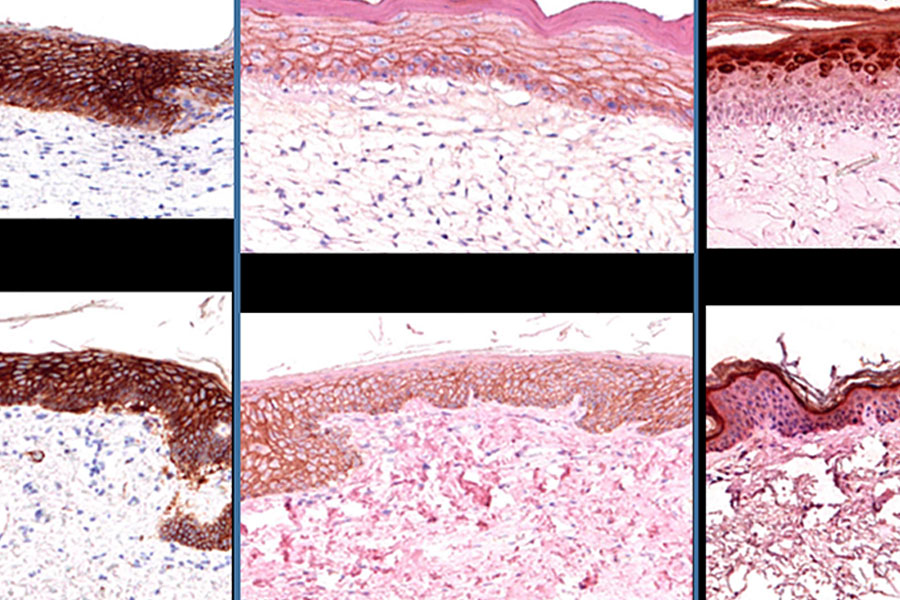
2013: A research collaboration agreement was signed between NIH — including NCATS and the National Eye Institute (NEI) — and Organovo, a company dedicated to pushing through the development of new therapies through the design and creation of functional human tissues using proprietary 3-D bioprinting technologies. As part of the partnership, NCATS installed an Organovo NovoGen MMX Bioprinter® to develop 3-D bioprinted retinal (jointly with NEI) and skin tissue.
2016: The 3-D Tissue Bioprinting program at NCATS received funding through the Cures Acceleration Network, which allowed the program to expand its bioprinting technical capabilities, create a histology core, and expand its microscopy imaging capabilities. It also enabled requests for applications (RFAs) to fund extramural labs to collaborate with NCATS to implement drug screens with 3-D bioprinted tissue models.
2017: NCATS published an RFA, RFA-TR-17-007, titled NCATS Pilot Program for Collaborative Drug Discovery Research Using Bioprinted Skin Tissue (U18). Two teams at Columbia University (Angela Christiano, Ph.D.) and Rockefeller University/New York University (Dan Gareau, Ph.D.) received funding through this opportunity.
2018: The 3-D Tissue Bioprinting Laboratory (3DTBL) was formally established in the Division of Preclinical development at NCATS.
2018: The 3DTBL received funding from NIH’s Helping to End Addiction Long-term (HEAL) initiative to develop biofabricated 3-D tissue models of nociception, opioid use disorder and overdose for drug screening.
2019: NCATS published an RFA, RFA-TR-19-020, titled Drug Screening With Biofabricated 3-D Skin Disease Tissue Models (U18). Two teams at Columbia University (Angela Christiano, Ph.D.) and University of Washington (Jia Zhu, Ph.D.) received funding through this opportunity.
2020: The 3DTBL received funding from the Coronavirus Aid, Relief, and Economic Security (CARES) Act (2020) to develop biofabricated 3-D tissue models for COVID-19 drug screening.
2021: NCATS published an RFA, RFA-TR-21-015, titled Intramural-Extramural Collaboration for Drug Screening with Biofabricated 3-D Disease Tissue Models (UH2/UH3). Two teams at the University of Pittsburgh (Dr. Mark Miedel) and University of Texas Medical Branch Galveston/Texas A&M (Drs. Ram Menon and Arum Han) received funding through this opportunity.