Matrix Screening Projects
Explore some of the projects NCATS experts are using matrix combination screening for to find drug combinations with the most potential to help patients.
Reasoned and data-driven clinical trials are built on a foundation of sound mechanistic rational and well-validated drug activity across disease models. The screening of approved and investigational drugs within cell-based phenotypic assays can provide evidence of drug activity that often starts the translational process. Drug screening can shed light on the activity of approved and investigational drugs from experiments involving tens of thousands of data points. Often, these experiments yield hundreds of seemingly high-value opportunities. Unfortunately, most in vitro surveys make compromises to deliver experimental feasibility at the expense of data quality. As a result, each agent is evaluated within a regulated assay format and on a prescribed timeline that are not typically customized to a drug’s unique mechanistic or pharmaceutical properties.
To combat the issues associated with assay standardization, we have constructed a chemogenomics and matrix screening pipeline that couples rapid data generation and analysis with an increasingly granular look at promising drug candidates and combinations that shed light on the most promising therapeutic opportunities. Although each program is unique, many of our projects progress through the following four experimental steps:
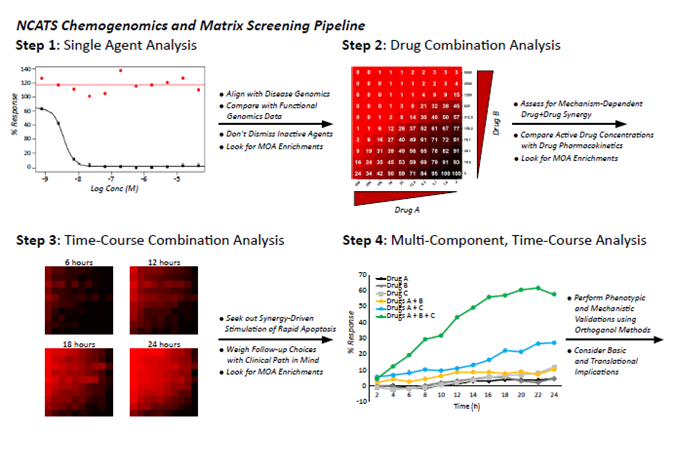
Explore selected matrix screening projects:
Combined Targeted Therapies for Lymphomas
The first published study using the NCATS matrix combination screening platform was an examination of the combination landscape of the BTK inhibitor ibrutinib in multiple cell models of lymphoma (Proc Natl Acad Sci U S A. 2014 Feb 11;111(6):2349-54). The Staudt and Wilson Labs (National Cancer Institute) used data from this study, alongside deep mechanistic and clinical knowledge of diffuse large B cell lymphoma (DLBCL), to design the TEDDI-R multi-component therapy for treating primary central nervous system (CNS) lymphoma (Cancer Cell. 2017 Jun 12;31(6):833-843.e5) and the VIPOR multi-component therapy for treating relapsed/refractory B-cell lymphomas (NCT03223610). The NCATS matrix combination screening platform has also contributed to basic research findings in lymphoma ranging from the validation of new drug targets (Cancer Discov. 2023 May 4;CD-22-1401) (Nature. 2018 Aug;560(7718):387-391) to the understanding of drug-resistant disease (Blood Cancer Discov. 2021 Sep 14;2(6):630-647). Our work with the Staudt Lab in lymphoma research represents one of the NCATS matrix combination screening platform’s longest collaborative partnerships.
New Therapies for Childhood Central Nervous System (CNS) Cancers
The NCATS matrix combination screening platform is proud to be a frequent contributor and collaborator with the pediatric cancer community. Our team has ongoing relationships with researchers tackling some of the most challenging childhood cancers, including rare malignancies involving the CNS. These include ongoing work in atypical teratoid/rhabdoid tumors (ATRT) (Resnick Lab, CHOP) and embryonal tumors with multilayered rosettes (ETMR) (Greenfield and Filbin Labs, Weill Cornell and Dana Faber, respectively). Several studies have revealed therapy concepts that have entered the clinic, including the use of intra-arterial chemotherapy for choroid plexus carcinoma (CPC) (Souweidane Lab, Weill Cornell) (J Control Release. 2023 May;357:580-590) and the CNS penetrant proteasome inhibitor marizomib for the treatment of diffuse intrinsic pontine gliomas (DIPG) (Warren and Monje Labs, Dana Faber and Stanford, respectively) (Sci Transl Med. 2019 Nov 20;11(519):eaaw0064).
Publications
- Thomas, A., Takahashi, N., et al. Therapeutic targeting of ATR yields durable regressions in small cell lung cancers with high replication stress. Cancer Cell Apr. 12, 2021.
- Lang, M., Schmidt, L.S., et al. High-throughput and targeted drug screens identify pharmacological candidates against MiT-translocation renal cell carcinoma. J Exp Clin Cancer Res. Apr. 25, 2023.
- Chory, E.J., Wang, M., et al. High-throughput approaches to uncover synergistic drug combinations in leukemia. SLAS Discovery. June 2023.
- Sun, Y., Baechler, S.A., et al. Targeting neddylation sensitizes colorectal cancer to topoisomerase I inhibitors by inactivating the DCAF13-CRL4 ubiquitin ligase complex. Nature Communications. Jun. 23, 2023.
- Nair, N.U., Greninger, P., et al. A landscape of response to drug combinations in non-small cell lung cancer. Nature Communications. Jun. 28, 2023.
- Kumari, A., Gesumaria, L., et al. mTOR inhibition overcomes RSK3-mediated resistance to BET inhibitors in small cell lung cancer. JCI Insight. Mar. 8, 2023.
- Yang, Y., Bolomsky, A., et al. Oncogenic RAS commandeers amino acid sensing machinery to aberrantly activate mTORC1 in multiple myeloma. Nature Communications. Sept. 17, 2022.


