Translational Science Resources
We offer a variety of resources that you can use to better understand translational science and the challenges it is addressing.
Our Translational Science Resources
Translational science is the field that generates scientific and operational innovations that overcome the long-standing barriers along the translational research pipeline. By advancing translational science, we can improve the process of turning research observations into health solutions and ultimately bring more treatments to all people more quickly. Explore these resources to learn more about translational science.
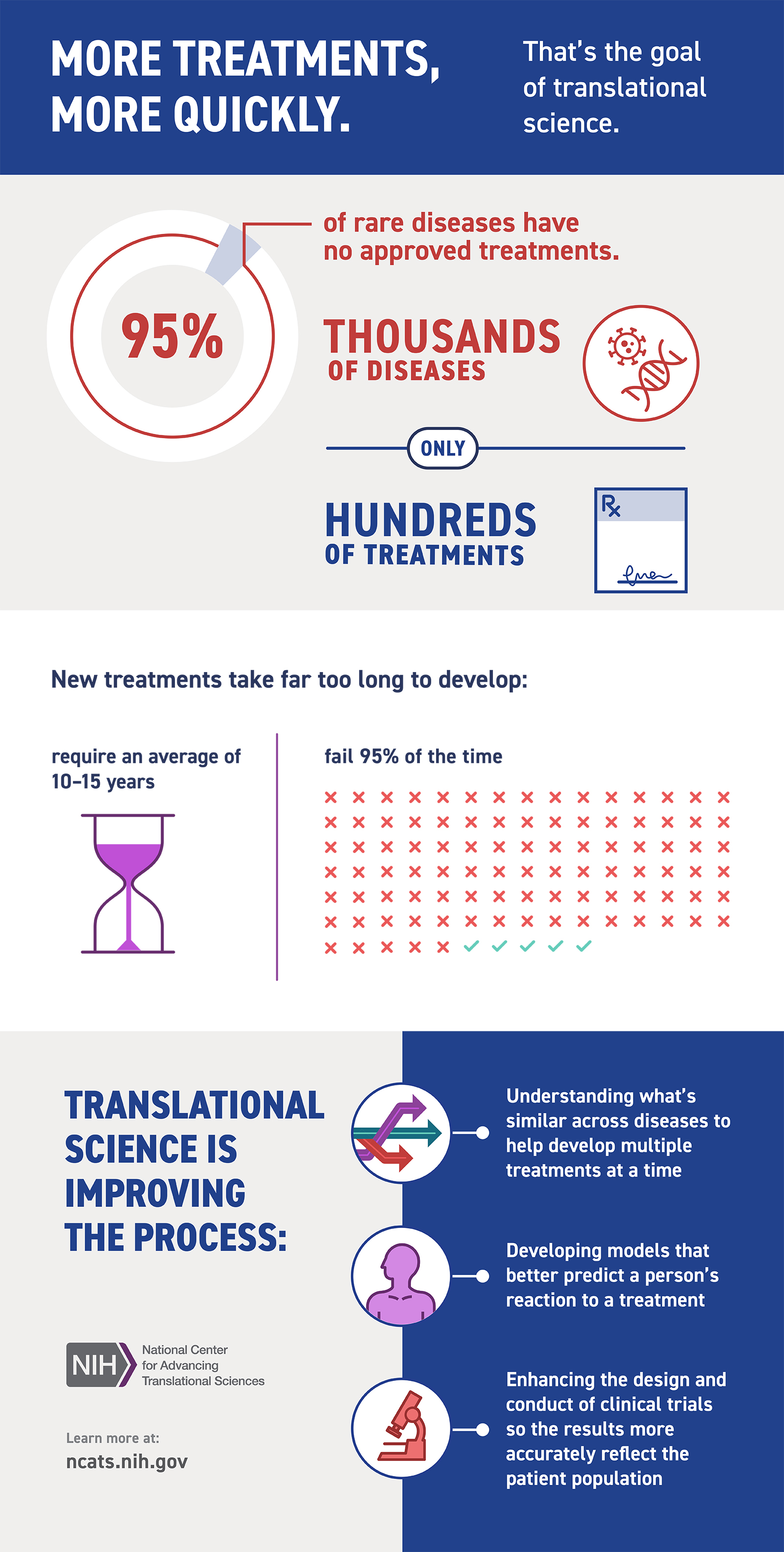
Translational Science Infographic
Developing new treatments can take a very long time. This infographic highlights some of the challenges as well as translational science approaches we’re using to overcome them.
Translational Science Infographic

Drug Discovery, Development and Deployment Maps
The Drug Discovery, Development and Deployment Maps (4DM) represent the modern therapeutic development process to more easily identify opportunities to expedite it. The maps provide a common framework for discussing the therapeutic development process and serve as an education tool for those who are new to it. They also can facilitate discussions on innovative solutions to existing bottlenecks.
Two versions of the 4DM are available: one for small molecules (Figure A), and another for biologics, using monoclonal antibodies as the representative therapeutic (Figure B). They illustrate some of the unique differences between the development of these products. Both files are licensed to the public under the Creative Commons Attribution-Share Alike International 4.0 (CC BY-SA 4.0) license, which allows use and adaption as long as the user provides attribution and shares any adaptations back to the public under the same license. Read more about the 4DM.
Translational Scientists
The Role of Translational Scientists
There is tremendous need for people to discover, develop and disseminate the next generation of science and technology to improve human health. Watch the video above to learn more about translational scientists.
Translational Science Characteristics
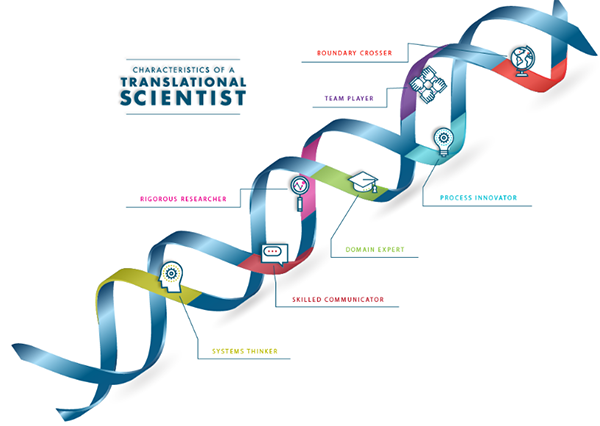
Translational scientists are innovative and collaborative, searching for ways to break down barriers in the translation process.