Multimedia
Visit our collection of videos or browse our fact sheets and infographics to learn more about us and download them to share our resources and activities with others.
Videos
Watch our collection of videos to learn more about NCATS and get a behind-the-scenes look at our research activities.
Videos
Our videos feature informative interviews with NCATS leadership and staff, inspiring patient stories, virtual lab tours, helpful grantee resources and more.
NCATS Infographics & Fact Sheets
View our fact sheets to learn more about us and download them to share our resources and activities with others.
3-D Tissue Models
NCATS designs and helps develop 3-D tissue models to help researchers better predict which promising treatments will move from the lab to success in clinical trials in people.
Read About 3-D Tissue Models (PDF - 222KB)
Assay Guidance Manual
The Assay Guidance Manual is a free, best-practices online resource devoted to the successful development of robust, early-stage drug discovery assays.
Read About AGM (PDF - 226KB)
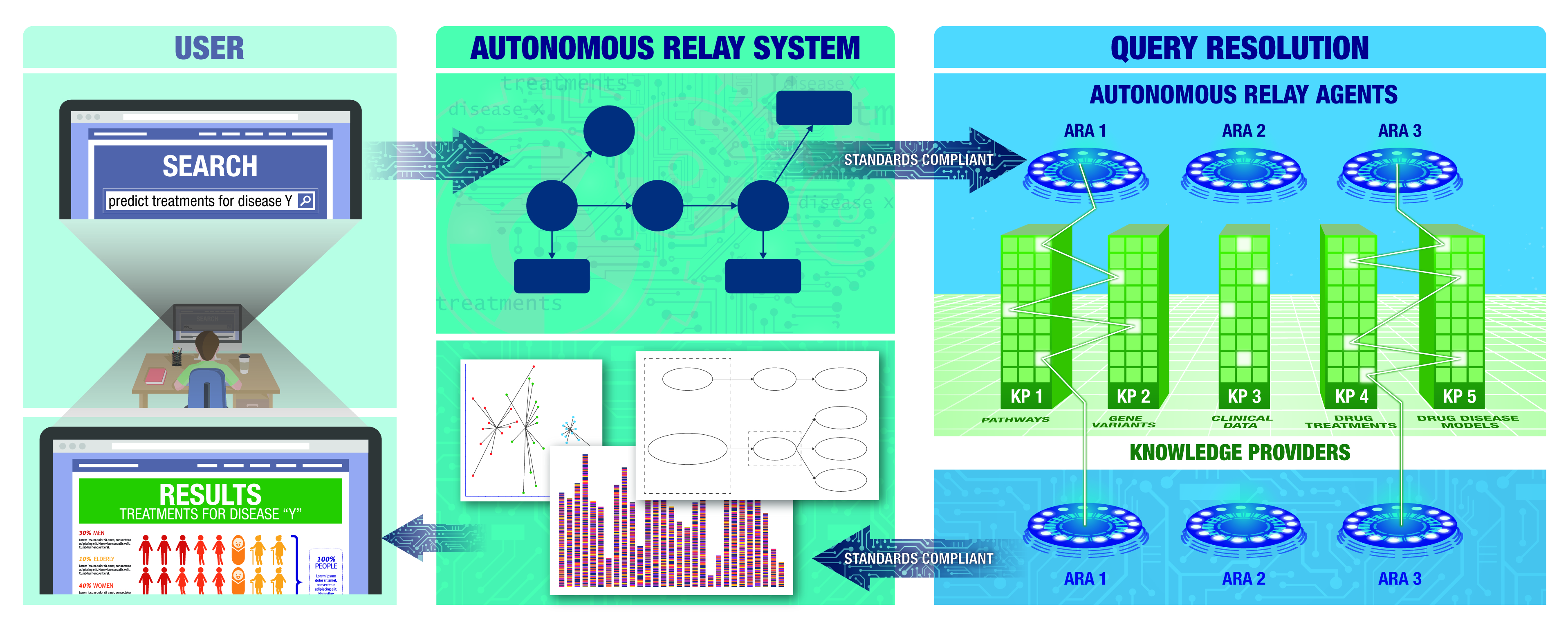
Biomedical Data Translator
This program brings together teams of experts from leading universities and research institutions to combine vast amounts of current medical research data and speed the development of new treatments.
Core Technologies
NCATS uses state-of-the-art resources and core technologies to enable the ongoing operation of all translational research activities happening at the Center.
Read About Core Technologies (PDF - 250KB)
National Clinical Cohort Collaborative (N3C)
The National Clinical Cohort Collaborative (N3C) maintains one of the largest collections of secure and deidentified clinical data in the United States for COVID-19 research.
Read About N3C (PDF - 282KB)
NCATS Drug Repurposing
Drug repurposing finds approved drugs and investigational compounds that might be able to treat other diseases and conditions. We use drug repurposing as a key strategy for bringing more treatments for all people more quickly.
Read About NCATS Drug Repurposing (PDF - 688KB)
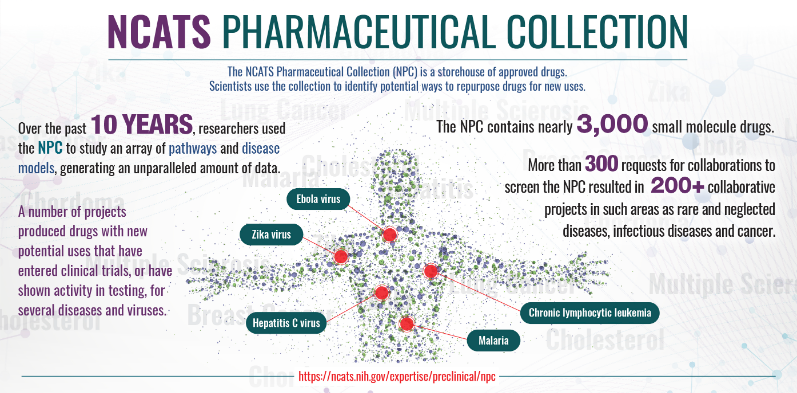
NCATS National Pharmaceutical Collection
The NCATS National Pharmaceutical Collection is a complete, publicly available collection of approved molecular parts for high-throughput screening. The collection provides a valuable resource for validating new models of disease and understanding the molecular basis of diseases and interventions better.
Learn More About the Pharmaceutical Collection (PDF - 451KB)
NCATS OpenData Portal
The OpenData Portal offers real-time information about how individual SARS-CoV-2 variants may respond to known therapeutics. It also focuses on NCATS’ broader drug repurposing findings and other pandemic threats.
Read About the OpenData Portal (PDF - 241KB)
NCATS Strategic Plan Framework
The Strategic Plan Framework includes 5 goals with supporting objectives to bring more treatments for all people more quickly.
View the NCATS Strategic Plan Framework (PDF - 1.22 MB)
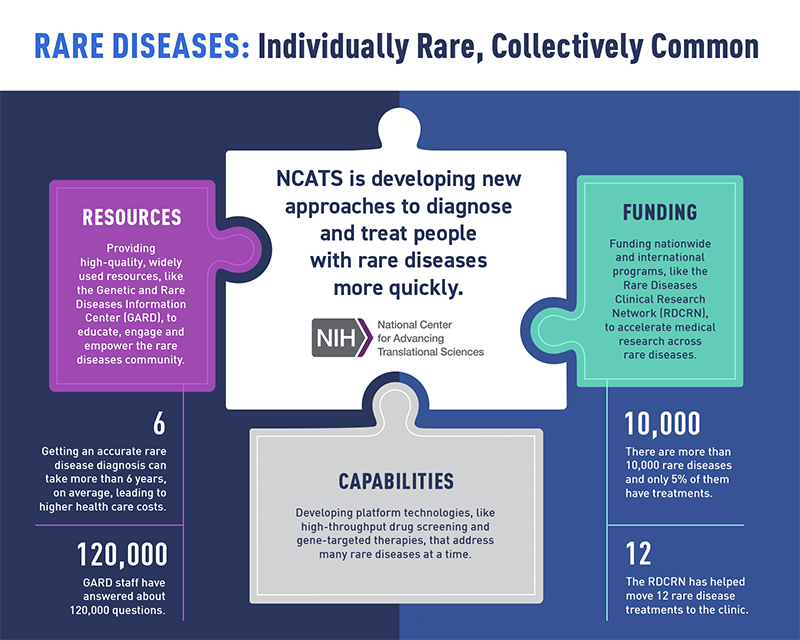
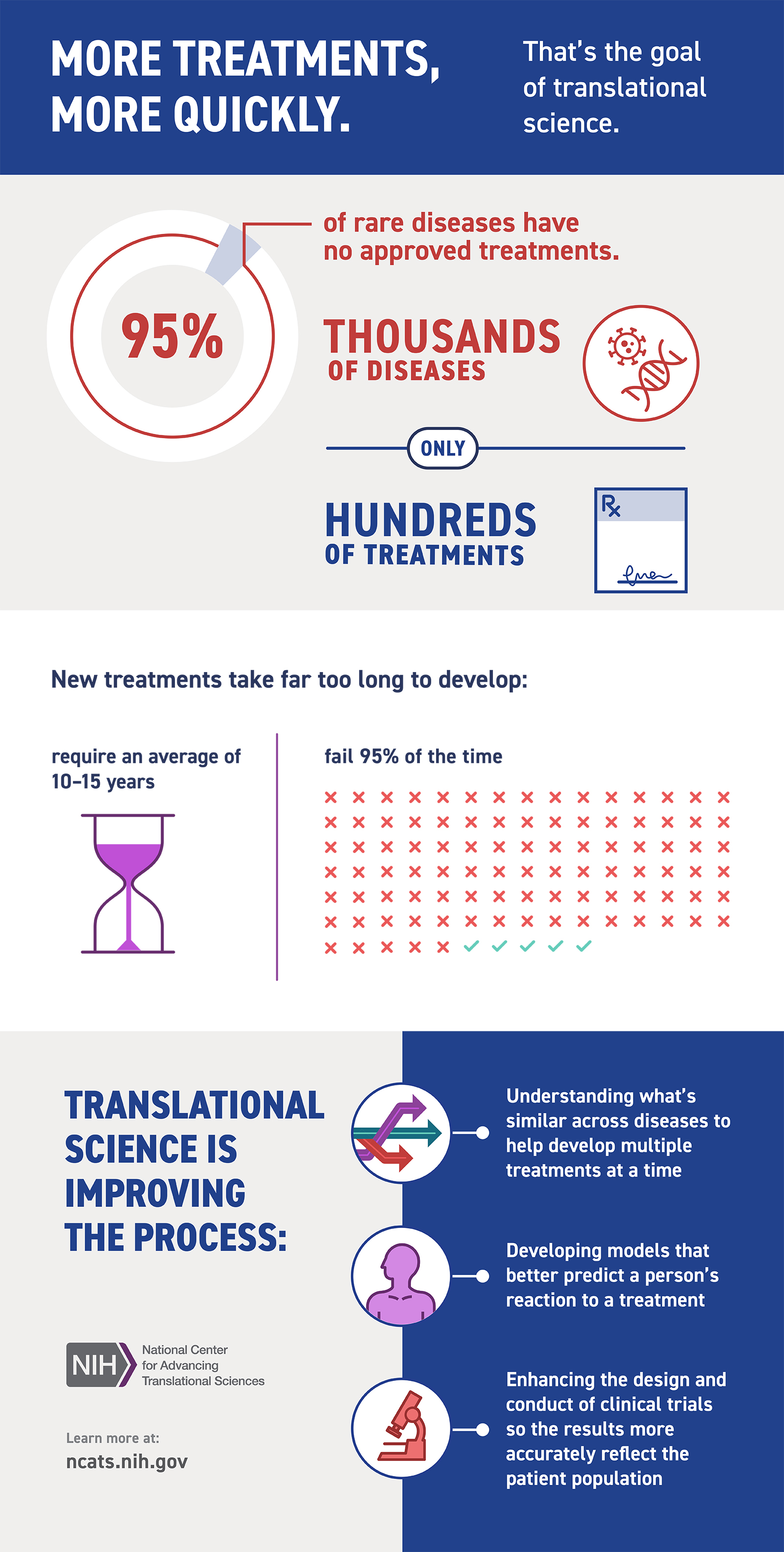
Rare Diseases
NCATS supports a range of innovative approaches for understanding and treating rare diseases, which affect millions of people in this country.
Read About Rare Diseases (PDF - 350KB)
Rare Diseases Overview
NCATS is developing new approaches to diagnose and treat people with rare diseases more quickly.
View the Rare Diseases Infographic (PDF - 110.14 KB)
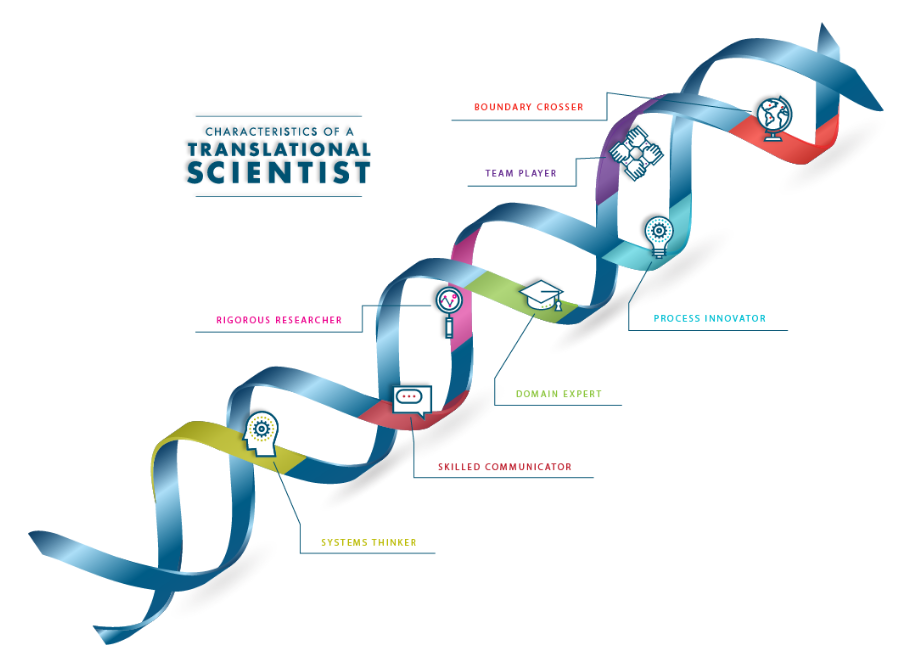
Seven Characteristics of a Translational Scientist
Translational scientists are innovative and collaborative, searching for ways to break down barriers in the translation process.
Learn More About the Characteristics of a Translational Scientist
Small Business Innovation
The Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs engage U.S. small businesses and research organizations in research and development that has the potential for commercialization and public benefit.
Read About SBIR/STTR Programs (PDF - 250KB)
Tissue Chips for Drug Screening
Developing new treatments for patients is a long and hard process. Often tests conducted in the early stages of research do not accurately predict how well a treatment will work or if it will cause any harm to humans. This leads to long delays and enormous costs while patients eagerly await effective treatments. The Tissue Chip for Drug Screening program at NCATS brings innovation to the table. By using specialized chips, this program is making exciting advancements in drug testing.
Translational Science Principles
Our seven translational science principles describe core approaches for effective translational science.
Translational Science
Translational science looks at the entire translational research ecosystem to identify common pitfalls and develop innovative solutions.
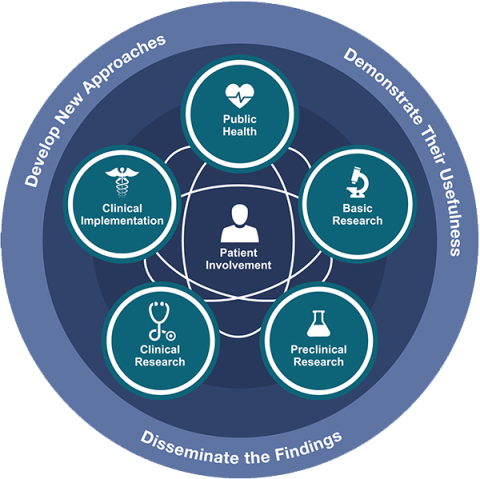
Translational Science Spectrum
The translational science spectrum covers all stages of research from basic research to public health.
Copyright and Reuse of Graphics
The NCATS website uses a mix of copyrighted and copyright-free graphics (including illustrations and photos). If you want to reuse a graphic, please follow these guidelines:
- Copyrighted graphics will usually be credited to individuals or organizations. Permission to reuse these must be negotiated directly with the creators, and not NCATS.
- Graphics explicitly credited to NCATS are copyright-free and may be used without our permission. Please credit the National Center for Advancing Translational Sciences as the source.
If you are not sure who created a graphic or have other questions about reusing a graphic on the NCATS website, email NCATS at ncatsinfo@nih.gov. Please include the URL and file name in your email.