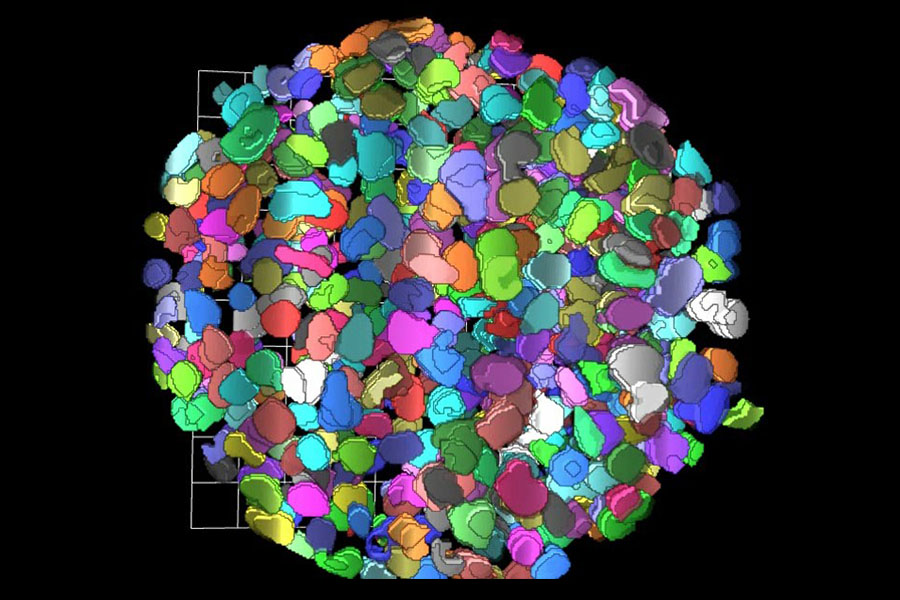
3-D Tissue Bioprinting
We use 3-D bioprinting to create models that mimic human tissues to speed drug discovery and development.
About the 3-D Tissue Bioprinting Program
About 90% of potential new drugs fail in clinical trials because they are not effective or they cause unexpected toxic effects. This failure happens in large part because the cell-based laboratory tests, called in vitro assays, and animal models used during the drug discovery and development process are too simplistic and do not accurately predict what will happen when the drug is given to humans.
There is a critical need for development of in vitro assays that can better predict the effectiveness and toxicity of potential drugs in humans. To address this need, we are applying the techniques of 3-D bioprinting to drug discovery and development. Through the process of 3-D bioprinting, researchers are developing tissue models that mimic the 3-D structure and organization of cells in the tissues of living organisms in microplate format for screening.
The Technology
Recent advances in the fields of engineering, biomaterials science, stem cell biology, physics and medicine have enabled the field of 3-D bioprinting of living tissues. Living cells and scaffolding materials can now be combined into complex 3-D functional tissues produced on microplates (tissue-in-a-well), which are used in preclinical drug testing. These tissues are created using either induced pluripotent stem cells (iPSCs) or primary cells taken directly from living tissue.
The goal of creating 3-D bioprinted human-like tissues in microplate format for screening is to provide physiological and pharmacological data that predict the effects of drugs better than data from traditional studies using 2-D models.
The Applications
At NCATS, the 3-D Tissue Bioprinting team is developing normal and disease tissue models for drug discovery and development. The team has created the infrastructure necessary to enable screening with 3-D bioprinting of tissue-in-a-well models.
The 3-D Tissue Bioprinting program builds on internal expertise in assay development for drug screening, automation technologies, stem cell biology, tissue engineering and advanced high-content microscopy. Learn more about the goals of the program.
The Collaborators
We are collaborating with academic investigators in the basic and clinical research communities who provide the disease and tissue physiology expertise necessary to develop and validate bioprinted tissues. These collaborators might also provide critical materials needed to study drug response, such as patient-derived iPSCs.
We also work with private-sector partners who provide technology and engineering solutions to enable this innovative work. The center is interested in establishing new collaborations with pharmaceutical and biotechnology companies to enable benchmarking and establish translational capacity for these tissue-in-a-well assays so that they can be integrated into the decision making process during the drug discovery and development pipeline.
3-D Bioprinting News

A New 3-D Skin Tissue Model Helps Identify Possible New Treatments for Herpes Simplex Virus
January 27, 2026 - NCATS News
- 3-D Tissue Bioprinting
The 3-D Tissue Bioprinting Program created a 3-D tissue model that mimics human skin to support an infectious disease study focused on identifying potential drug treatments for herpes simplex virus.
Read ArticleNIH Launches Organoid Development Center
September 26, 2025 - Media Coverage
- 3-D Tissue Bioprinting
- Tissue Chip for Drug Screening
NIH’s $87 Million Nonanimal Testing Project
September 25, 2025 - Media Coverage
- 3-D Tissue Bioprinting
- Tissue Chip for Drug Screening
NIH Establishes Nation's First Dedicated Organoid Development Center to Reduce Reliance on Animal Modeling
September 25, 2025 - Grantee/Partner News
- 3-D Tissue Bioprinting
- Tissue Chip for Drug Screening
Bypassing the Brain
Olive Jung, a graduate student at NCATS in the NIH’s Intramural Research Program, explains in this video (video length: 2:39) how she is developing a 3-D model that acts like the blood-brain barrier to allow vital nutrients and oxygen into the brain while keeping out harmful substances as well as beneficial drugs. The 3-D model could help accelerate therapeutic development by allowing scientists to test whether new medications are able to get through the real blood-brain barrier. A version of this video with audio description (video length: 2:39) is available.
Work With Us
We are interested in establishing new collaborations with pharmaceutical and biotechnology companies. These efforts would focus on benchmarking and establishing translational capacity for tissue-in-a-well assays to be integrated into the discovery and development process for new therapies. Contact Marc Ferrer if you are interested in working with the 3-D Tissue Bioprinting team.
Related Research

Tissue Chip for Drug Screening Technology
This program develops and tests the use of human cell-based models for predicting drug safety and toxicity before clinical testing in people.

Stem Cell Translational Laboratory
This NCATS lab develops methods and standards to bring stem cell technology closer to clinical application, drug discovery and regenerative medicine.

Assay Development and Screening Technology
Our scientists explore and develop new ways to quickly test and screen potential drug candidates, often in collaboration with disease foundations.