An Organ-on-a-Chip Model of ALS Will Help Uncover Early Disease Characteristics and Find Potential Therapies
August 6, 2025
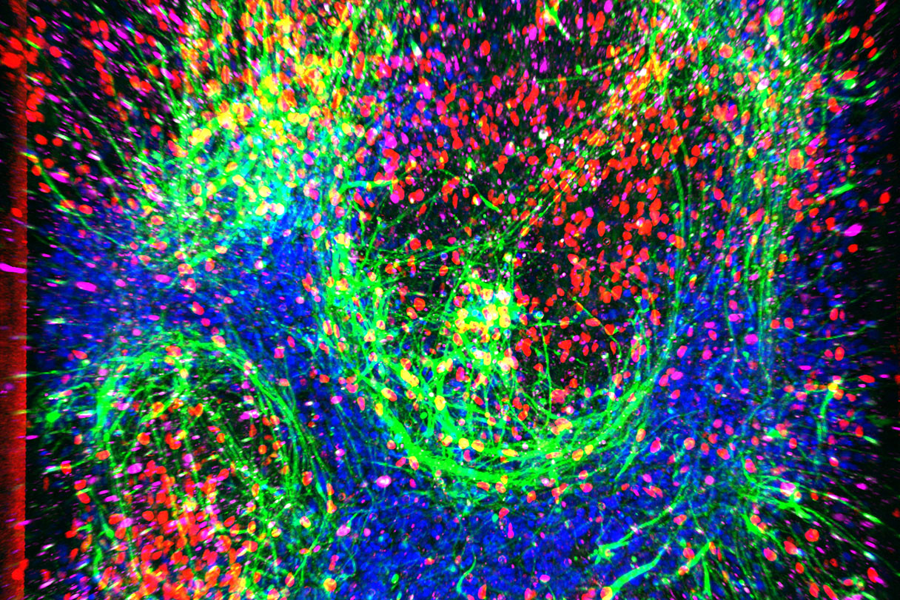
A neurodegenerative disorder known as amyotrophic lateral sclerosis (ALS) causes paralysis and is usually fatal within two to five years. Motor neurons (MNs) send signals from the brain and spinal cord to the body; they control normal functions, such as breathing, talking and walking. The hallmark feature of ALS is permanently damaged MNs. About 90% of ALS cases have an unknown origin, and the disease is not passed down from parents. Part of what’s hindered our understanding of ALS is that current cell culture models of ALS are limited and do not effectively mimic key traits of human physiology.
A recent study at Cedars-Sinai supported by NCATS’ Tissue Chip for Drug Screening program focused on creating a three-dimensional cell culture model — an organ-on-a-chip — of ALS. Researchers have called it the spinal cord chip (SC-chip). The SC-chip grows MNs and endothelial cells (cells that line blood vessels and regulate blood flow) together in parallel chambers of a miniaturized chip known as a microfluidic device. These chambers are separated by a membrane that allows fluid to pass through, enabling nutrients to be absorbed and cell waste to be removed, which keeps the cells alive and mimics the human blood-brain barrier. Researchers isolated cells that become MNs from healthy (control) patients and from ALS patients to grow in the SC-chips. Compared with standard two-dimensional cell cultures of MNs, SC-chips provide an environment for MNs to closely mimic what is seen within a living organism, which makes them useful for advancing human health. SC-chip analysis showed that MNs isolated from ALS patients had unique changes in the amount of molecules related to neurofilaments, glutamatergic signaling, and synapse activity compared with MNs from control patients.
Using the SC-chip, future studies will explore the underlying causes of ALS, advance understanding of the disease’s progression, and provide a human-relevant model for drug screening of possible therapies for ALS patients. Danilo A. Tagle, Ph.D., director of NCATS’ Office of Special Initiatives, stated, “The result from this important study lays the groundwork for investigating the role of increased glutamate signaling in the gradual death of motor neurons in ALS, and provides opportunities to screen for interventions.”
The results appeared in Cell Stem Cell.
Related Content
Tissue Chip for Drug Screening
This program funds the development and testing of models built from human cells for predicting drug safety and toxicity before clinical testing in people.
Protein in the Blood Can Help Predict ALS Survival and Decline, Play Role in Developing Treatments
NIH-supported researchers showed how the level of a protein found in blood might be used in developing new drugs to treat ALS.