NCATS Collaborations Lead to Potential New Drug Combinations for Childhood Cancers
Translational Science Highlight
- NCATS scientists are bridging translational gaps in moving promising compounds and drugs closer to clinical trials for several childhood cancers that lack effective treatments. In addressing this unmet need, the NCATS team is building expertise in drug interactions that will help it continue to foster new collaborations to tackle translational challenges.
In the past several decades, researchers have made great gains in treating children who have cancer, particularly those with blood cancers such as leukemia and lymphoma. But finding effective therapies for certain cancer types has been more difficult.
To help address this challenge, a team of researchers in NCATS’ Chemistry Technology and Matrix Screening programs is rethinking treatment approaches for several difficult-to-treat childhood cancers. Using NCATS’ matrix combination screening capabilities, team leader Craig Thomas, Ph.D., and his colleagues are taking some of the guesswork out of drug discovery. The team uses this screening technique to rapidly test the effects of thousands of different drug combinations on key disease processes. Scientists can examine the most effective combinations, find the best doses of each drug and learn more about their effects on cells. The NCATS team and its collaborators focus on drug combinations that work in synergy and that can be tested in laboratory models and, ultimately, people.
“The matrix screening technology is a perfect example of how translational science should work,” said Anton Simeonov, Ph.D., NCATS scientific director. “This is the nature of what NCATS does — finding answers and enabling others to develop therapies that can be tested through clinical trials to improve patients’ lives.”
Collaborating at NIH

NCATS’ chemistry technology lead Craig Thomas, Ph.D. (left), discusses the results of a drug screen with scientists Michele Ceribelli, Ph.D., and Scott Hoyt, Ph.D. (Daniel Soñé Photography)
One disease under study is Ewing sarcoma, a rare bone and soft tissue cancer that mostly affects teenagers and young adults. Although usually initially treatable, like many cancers, it is more difficult to cure if it returns.
Christine Heske, M.D., who treats children and young adults with Ewing sarcoma and studies the disease at NIH’s National Cancer Institute (NCI), recently teamed with the NCATS group to show that Ewing sarcoma cells were particularly sensitive to nicotinamide phosphoribosyltransferase (NAMPT inhibitors), a class of drugs that has been tested only in adult cancer types. These drugs block the activity of an enzyme that is important for the metabolic function of cells. Cancer cells seem to rely on this enzyme more heavily than normal cells do.
Using matrix screening, NCATS researchers demonstrated how NAMPT inhibitors worked together with poly (ADP-ribose) polymerase (PARP) inhibitors, a class of drugs approved by the Food and Drug Administration to treat ovarian and breast cancers, in laboratory cell models of Ewing sarcoma. Meanwhile, Heske’s team at NCI showed the effectiveness of the drug combination in mouse models of the cancer. The researchers reported their results in the Dec. 1, 2017, issue of Clinical Cancer Research.
“Seeing which compounds and drugs have enhanced anticancer activity together may allow us to lower doses of each in treating patients,” Heske noted. She is hopeful the work eventually will lead to testing in patients.
In another collaborative effort, NCATS scientists, NCI colleagues and an international team of researchers examined rhabdomyosarcoma (RMS), a rare muscle cancer that often affects children and adolescents. There are two main forms of the disease in children, and both can have difficult treatment paths.
One form of RMS is caused by a genetic mix-up in which DNA changes places on a chromosome, a structure that houses genetic material inside a cell. This error prompts cells to mistakenly grow out of control — a hallmark of cancer. The other major form of RMS works through a different biological route.
The collaborators screened compounds and drugs for their activity against both types of RMS. They found combinations of compounds that were more active against each form of cancer.
The first set of results, published in the August 2017 issue of Cancer Discovery, showed that a group of compounds called BRD4 inhibitors was more effective against the form of RMS caused by the genetic switch. The scientists further reported in the July 4, 2018, issue of Science Translational Medicine that the other form of RMS seemed more susceptible to a combination of compounds that worked against a completely different target.
“These screens identified important vulnerabilities in each form of RMS because of each one’s unique sensitivities to certain classes of drugs,” said NCI’s Marielle Yohe, M.D., Ph.D., a co-author on both studies. “This insight can help us determine which drugs are most effective.”
One Partnership Leads to Another
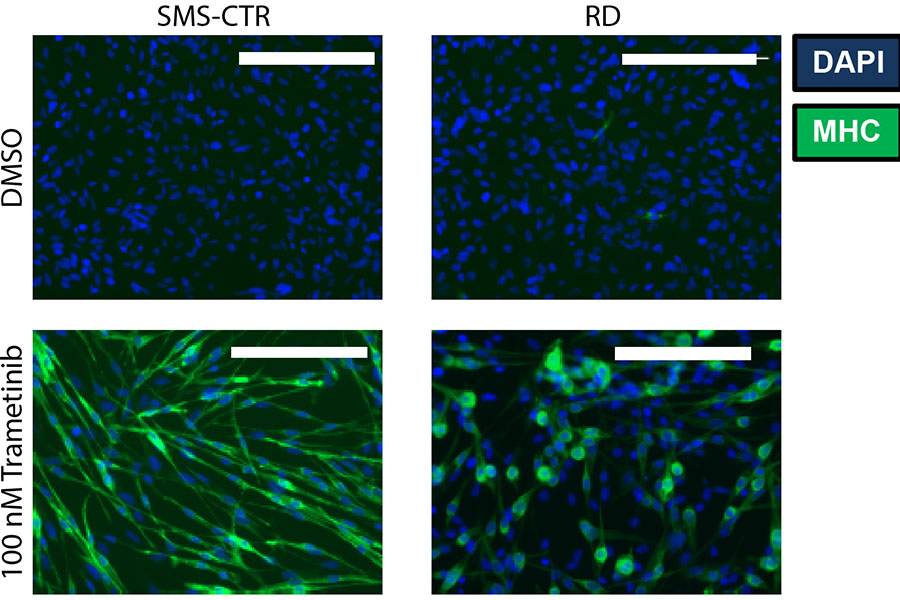
Treatment with the drug trametinib can help immature muscle cells, which characterize the cancer rhabdomyosarcoma, develop into mature cells (shown in the bottom two panels in green). In the top two panels, immature cells (blue) are treated with a control drug, with no effect. (Reprinted with permission from Yohe, et al., Sci. Transl. Med. 10, eaan4470 (2018))
For the past three years, NCATS has collaborated with Stanford University neuro-oncologist Michelle Monje, M.D., Ph.D., to study a rare childhood brain tumor called diffuse intrinsic pontine glioma (DIPG). These aggressive, hard-to-treat tumors are the leading cause of brain cancer death among U.S. children.
Monje and her team had discovered that a drug called panobinostat showed activity against DIPG cells. Buoyed by some preliminary successes, Monje wanted to look at how a range of compounds and combinations affected the cancer.
In 2015, NCI’s Katherine Warren, M.D., who was working with Monje, spearheaded a collaboration with NCATS. Since then, Monje, Thomas and their teams have used NCATS’ matrix screening technology to examine single compounds and combinations of compounds against DIPG patient cells. While they continue studies with panobinostat, they also are confronting another challenge: finding effective treatments that cross the brain’s protective blood-brain barrier.
“We’re examining possible drug targets identified through the NCATS screens and are working to bring the most promising combinations to clinical trials,” Monje said. “The pieces are beginning to fall into place.”
Honing Translational Expertise
These collaborative efforts with disease experts enable NCATS scientists to build their translational knowledge portfolio. By participating in dozens of such studies, NCATS is gaining vast knowledge about how drugs can interact, which is a complication associated with many diseases and disorders. NCATS scientists are discovering that some combinations nearly always work in tandem against certain types of cancers, especially those combinations that affect processes cancer cells need to survive.
“We want to learn the broader trends and build institutional expertise,” Thomas said. “If we understand why some molecules and combinations are more or less effective and what variations in a person’s genome drive certain responses, we can make better decisions as we translate basic science discoveries to improve human health.”
Posted December 2018


