NIH-Supported Research Helps Advance a Potential Rare Disease Gene Editing Treatment into Clinical Trials
Translational Science Highlight
- NIH's Rare Diseases Clinical Research Network-supported research helps overcome a translational roadblock by charting how several rare, inherited disorders progress in patients over time. This information enabled a gene editing therapy for a group of rare diseases with few treatments to advance to clinical trials.
Lysosomal diseases affect about one in 6,000 people worldwide. These disorders are characterized by an abnormal buildup of various toxic materials in the body’s cells — the result of enzyme deficiencies. A pair of rare, inherited lysosomal diseases, Hurler and Hunter syndromes, can cause enlarged facial features, heart and other organ damage, bone and joint problems, developmental delays, brain damage, and early death.
In February 2013, scientists from Sangamo BioSciences, Inc. (now Sangamo Therapeutics, Inc.) asked University of Minnesota clinical biochemical geneticist Chester Whitley, M.D., Ph.D., to help them find out whether their novel gene editing technique could prevent or reverse the two syndromes. If the therapy proved safe and effective in animals, then clinical trials with patients could follow.
With support from the National Institutes of Health (NIH), initially through its Eunice Kennedy Shriver National Institute of Child Health and Human Development, Whitley and colleague R. Scott McIvor, Ph.D., had developed extensive experience in using mouse models to study several types of lysosomal diseases — including Hurler and Hunter syndromes — and their effects on the brain, in addition to gene therapy approaches.
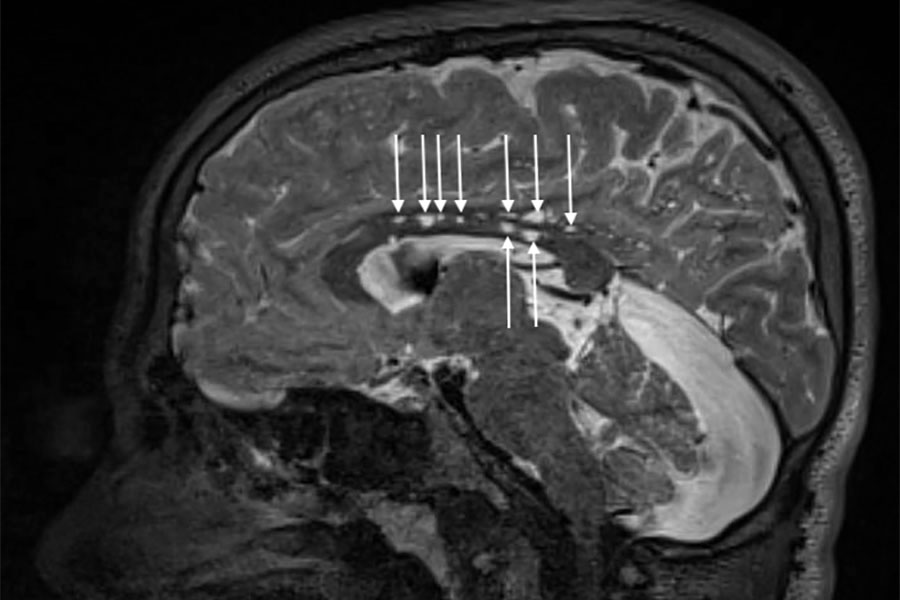
Magnetic resonance imaging reveals the brain’s structure. In this image, enlarged spaces (arrows) around blood vessels in the brain of a patient with Hunter syndrome, a neurodegenerative disease, show a physical effect of the disorder. (University of Minnesota Photo/Igor Nestrasil, M.D., Ph.D. and Carol Nguyen)
Subsequently, as part of the National Center for Advancing Translational Sciences (NCATS) Rare Diseases Clinical Research Network (RDCRN)-funded Lysosomal Disease Network (LDN) — which included support from the National Institute of Neurological Disorders and Stroke and the National Institute of Diabetes and Digestive and Kidney Diseases — Whitley and LDN scientists conducted clinical studies of affected children and adults. The research included natural history studies, which revealed how these diseases progress over time. Knowledge of a disease’s natural history is essential to establishing the scientific foundation for developing effective treatments.
“NIH support has been crucial to understanding the underlying biology and natural progression of lysosomal diseases,” said Anne Pariser, M.D., director of NCATS’ Office of Rare Diseases Research. “This support also helps scientists establish collaborations among specialized clinical sites, setting the stage for innovative research.”
Standard therapies for these disorders, such as injecting the missing enzyme directly into the body and bone marrow transplantation, have limited effectiveness and frequently have significant side effects.
Working with Sangamo researchers, Whitley and LDN Fellow Li Ou, Ph.D., found that a gene editing technique could prevent or reverse Hurler syndrome in some tissues in mice with the disease. When the researchers evaluated the technique’s effects in a learning behavior test, the results in treated mice suggested that the therapy reached the brain. Along with similarly promising findings in mice with Hunter syndrome, the stage was set for the next step: testing in patients.
In November 2017, Sangamo made headlines when scientists made the first attempt to edit a gene inside the human body in a patient with Hunter syndrome.
RDCRN: Collaborative Approaches Address Roadblocks to Rare Diseases Research
Establishing the LDN was a watershed moment for many who study lysosomal diseases. RDCRN support enabled previously separate groups of lysosomal disease researchers from several institutions to pool patient data, experiences and expertise. With larger numbers of lysosomal disease patients available, the researchers could conduct the important natural history studies that examined how patients fared over time, with and without treatment.
Such studies enabled scientists to develop cognitive tests and brain imaging tools. RDCRN support eventually led to the creation of a national network of 10 centers for brain testing and imaging for patients with lysosomal diseases.
Patients and patient advocacy groups are part of the LDN, as both supporters and participants, and share their perspectives on living with lysosomal diseases. “We work with patients and families to figure out how best to design research studies that will help meet their needs,” Whitley said. “They let us know what we’re doing well and where we’re falling short.”
Cyndi Frank is a patient with Gaucher disease, a related lysosomal disease. She has participated in several trials over the past 40 years, including an LDN study of a possible drug treatment in the brain, and said it was vital that researchers listen to patients. “Doctors aren’t patients, and they can miss things,” she said. “They are worrying about treating the underlying disease problems, some of which can be life threatening. But many aspects of living with a disease can get overlooked. It can be difficult to appreciate, for example, what it means to endure almost disabling fatigue.”
The LDN’s Latest Role
Whitley and LDN researchers have worked with Sangamo to develop the current clinical trial and recruit participants. Some of the centers involved in the Sangamo trial are part of the LDN, including the UCSF Benioff Children’s Hospital Oakland and the University of Minnesota.
Sangamo and LDN scientists will gauge the safety of the gene editing technique in patients and will also watch for signs of effectiveness by using LDN-developed tools, including brain imaging. In addition, LDN investigators will help study and evaluate the effects of the gene editing therapy on patients’ thinking and learning abilities. In the meantime, LDN researchers continue to examine how these diseases and treatments affect the brain over time.
“These kinds of studies will help clinicians tailor treatments,” added LDN investigator Elsa Shapiro, Ph.D.. “There are many drug and biotechnology companies working in this field right now that are relying on this kind of information coming out of the LDN.”
Posted May 2018


