Harnessing the Immune System to Help Treat Cancer
Juan Vasquez, M.D.
https://medicine.yale.edu/profile/juan_vasquez/
Assistant Professor, Department of Pediatrics (Hematology/Oncology), Yale School of Medicine

Juan Vasquez, M.D.
I first became interested in biomedical research as an undergraduate, while volunteering for an afterschool swim program for children with asthma. This experience led me to work as a research assistant, examining the psychosocial, socioeconomic and cultural factors contributing to asthma disparities between Latino and Caucasian children. Ultimately, I decided to pursue a medical career, with the intention of becoming a general academic pediatrician.
During my pediatric residency at Brown University, my career goals shifted when I rotated on the hematology/oncology service. I became fascinated with the possibility of harnessing the immune system to help treat cancer while caring for a patient with neuroblastoma undergoing immunotherapy with a chimeric antibody. That is when I decided to pursue a career as a clinician-scientist. My long-term goal is to become an independent physician-scientist focused on developing immunotherapies to treat pediatric tumors.
After residency, I began my pediatric hematology/oncology fellowship at Yale University. It was during my fellowship that I began cancer immunology research under the mentorship of Dr. Kavita Dhodapkar. Through this mentored research experience, I learned the foundations of human immunology and laboratory research methods. I completed a project studying the immune response to SOX2, an embryonal stem cell antigen, in pediatric glial tumors. I also learned how to use single-cell mass cytometry to show that pediatric glial tumors are enriched for T-cells that display a tissue-resident memory phenotype and PD-1 expression.
Following the fellowship, I remained at Yale as an instructor, with a plan to gain additional research training. Around this time, my mentor moved to a different institution, but I had not yet gained the skills and funding needed to be an independent researcher. It was during this precarious interval that I applied for and received an NCATS diversity supplement through the Yale Clinical and Translational Science Awards (CTSA) Program grant. This supplement was pivotal in providing me with the support I needed to maintain my protected research time and to continue mentored research training with Dr. Ranjit Bindra. I also have been able to obtain additional translational research skills, such as developing animal models for preclinical drug testing and subsequently designing bench-to-bedside, early-phase clinical trials.
The additional mentored research training from this supplement also allowed me to acquire preliminary data for extramural grant applications. I have since been awarded a career development grant from the Harold Amos Faculty Development Program and have secured a position as an assistant professor in the Yale Section of Pediatric Hematology/Oncology. I have no doubt that the funding offered by the NCATS CTSA Program diversity supplement was pivotal in my success, and I strongly encourage all aspiring clinician-scientists from underrepresented backgrounds to take advantage of this program.
Current Research
Immune checkpoint inhibitors are a promising new cancer therapy, but only a subset of patients will respond. Tumors with a high mutational burden and more infiltrating immune cells are more likely to respond. However, most pediatric cancers, particularly brain tumors, are non-inflamed, with a low number of mutations. My first project focused on characterizing the immune landscape of pediatric brain tumors using single-cell mass cytometry. I demonstrated that pediatric glial tumors are enriched for tissue-resident memory T-cells, which co-express multiple inhibitory checkpoints, including PD 1. Additionally, I studied the expression of and immune response to SOX2, a shared tumor antigen, in pediatric glial tumors.
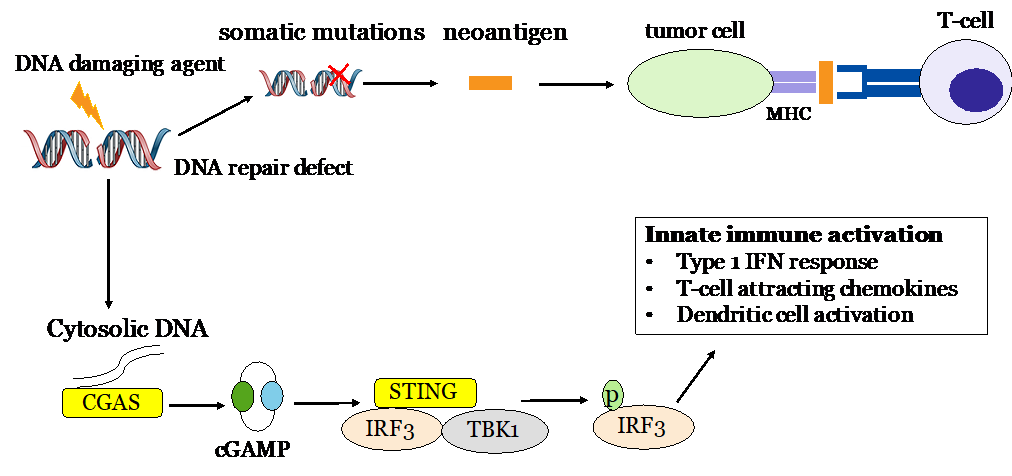
My current project focuses on the interface of DNA repair and the anti-tumor immune response. Dr. Bindra recently discovered that the accumulation of oncometabolites from mutations in citric acid cycle genes induces homologous recombination defects. My research is focused on identifying methods by which oncometabolite-induced DNA repair defects can be exploited with DNA repair inhibitors and DNA-damaging agents to sensitize glial tumors to immune checkpoint inhibitors.