Platform Approaches Pivot to COVID-19 Efforts
A key principle in translational science is to develop solutions that address common and persistent challenges in research, thus increasing the pace of testing and treatment for many diseases at a time. At NCATS, this strategy has led to crosscutting approaches that can pivot to address urgent public health needs.
Platform Approaches Pivot to COVID-19 Efforts

NCATS biologist Michele Ceribelli, Ph.D., prepares samples for a high-throughput flow cytometry analysis in a 384 well plate. (Daniel Soñé Photography, LLC)
Assay and Compound Libraries
It can take scientists more than a decade to turn a promising compound into an approved drug for patients. Repurposing existing drugs for new uses can potentially speed up the drug development process. Our drug repurposing programs support research at NIH and beyond to improve drug repurposing, including for projects focused on COVID-19. Scientists can collaborate with NCATS to use its drug-repurposing resources, including assay and compound libraries.
- A team, including our researchers and those of Cornell University, used our pharmaceutical collection to rapidly test each of its 2,678 approved or investigational compounds to identify those that inhibit SARS-CoV and MERS-CoV particles from entering mammalian cells. Of the six compounds that showed potential effectiveness against SARS-CoV-2 infection in a live cell assay, the most potent included a natural compound, a breast cancer drug and a lung cancer drug.
Read their October 2020 article in ACS Pharmacology & Translational Science. - Our scientists and collaborators at the Southern Research Institute, Naval Research Laboratory and Scripps Research Institute used our compound library to identify several compounds and drugs that inhibited a cell recycling process and could protect cells against SARS-CoV-2. Through a series of tests, the researchers found the compounds also blocked a process that viruses use to infect cells. The investigators suggested such inhibitors could someday be part of an anti-SARS-CoV-2 drug cocktail.
Learn more in this September 2020 story.
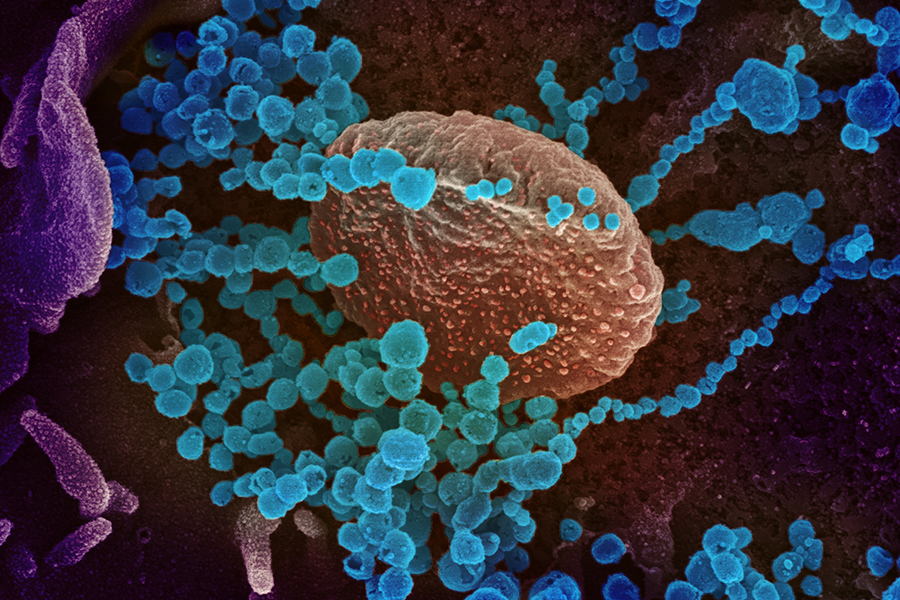
This scanning electron microscope image shows SARS-CoV-2 (round blue objects) emerging from the surface of cells cultured in the lab. SARS-CoV-2, also known as 2019-nCoV, is the virus that causes COVID-19. (NIAID)
Building a Portfolio of Promising Antiviral Therapies for Future Pandemics
We ring our proficiency in drug discovery and development to fill a critical role in the Antiviral Program for Pandemics (APP). The APP aims to develop treatments for SARS-CoV-2 and to speed up the development of a portfolio of promising antivirals that can quickly pivot to combat future pandemics. As part of the APP, we support early discovery and preclinical development by applying its expertise and cutting-edge resources in a variety of ways, including developing novel assays for viral targets and using high-throughput screening to identify potentially effective compounds.
Learn more about NCATS’ role in accelerating antiviral development.
Empowering Rapid Data Sharing
As soon as COVID-19 was identified, researchers all over the world — including at NCATS — started looking for drugs that could attack the virus in different ways. They developed assays, or tests, to screen existing drugs for their therapeutic potential. To allow the community to speed up the worldwide search for possible therapies, we launched the OpenData Portal with information on more than 10,000 compounds screened in its SARS-CoV-2–related assays. Data sets and the assay protocols used to generate them are posted as the screens are completed. The screening data, which include both positive and negative results, can be viewed, sorted, searched and exported from the Portal website. The website also includes detailed information on drug targets and mechanisms of drug action. This is the first time any organization has made this kind of drug repurposing information publicly available.
Visit the OpenData Portal.
Tissue Chips to Test the Safety and Efficacy of Antiviral Therapies
Approximately 30% of promising medications fail in human clinical trials because they are determined to be toxic despite promising preclinical studies in animal and cell models. Tissue chips — small, 3-D bioengineered devices that model human organs — could improve drug testing and development by allowing scientists to predict more accurately how safe and effective drugs are before they are tested in people. Researchers are investigating whether tissue chips could help speed up the development of COVID-19 treatments. For example, an NCATS-supported research team that developed a lung-on-a-chip to study and treat influenza infection used it to test existing antiviral therapies for their potential to treat COVID-19.
Read their May 2021 article in Nature Biomedical Engineering to learn more about this research.
Innovating Solutions to Speed Diagnostics and Treatments
Our Clinical and Translational Science Awards (CTSA) Program exists to support a network of academic research institutions in their development of innovative solutions, including diagnostics and treatments, and CTSA Program institutions quickly used their expertise and resources to address this public health emergency. The University of Rochester CTSA Program institution is one example. In April 2020, it rapidly adapted previous research to develop a potential diagnostic test that would require just a few drops of blood to detect the fast-spreading COVID‑19 virus. This research grew out of a past project in which a finger-stick test was developed that can detect immunity to more than 50 strains of the flu from just a few drops of blood.
Read this April 2020 story from the University of Rochester Clinical & Translational Science Institute to learn more.


