Accelerating Clinical and Translational Science Research Through Collaboration and Innovation
Explore the Collaborative Innovation Awards (CCIA) within the Clinical and Translational Science Awards (CTSA) Program, at NCATS. Learn about projects driving collaborative research, fostering innovation, and speeding the translation of scientific discoveries into health solutions.
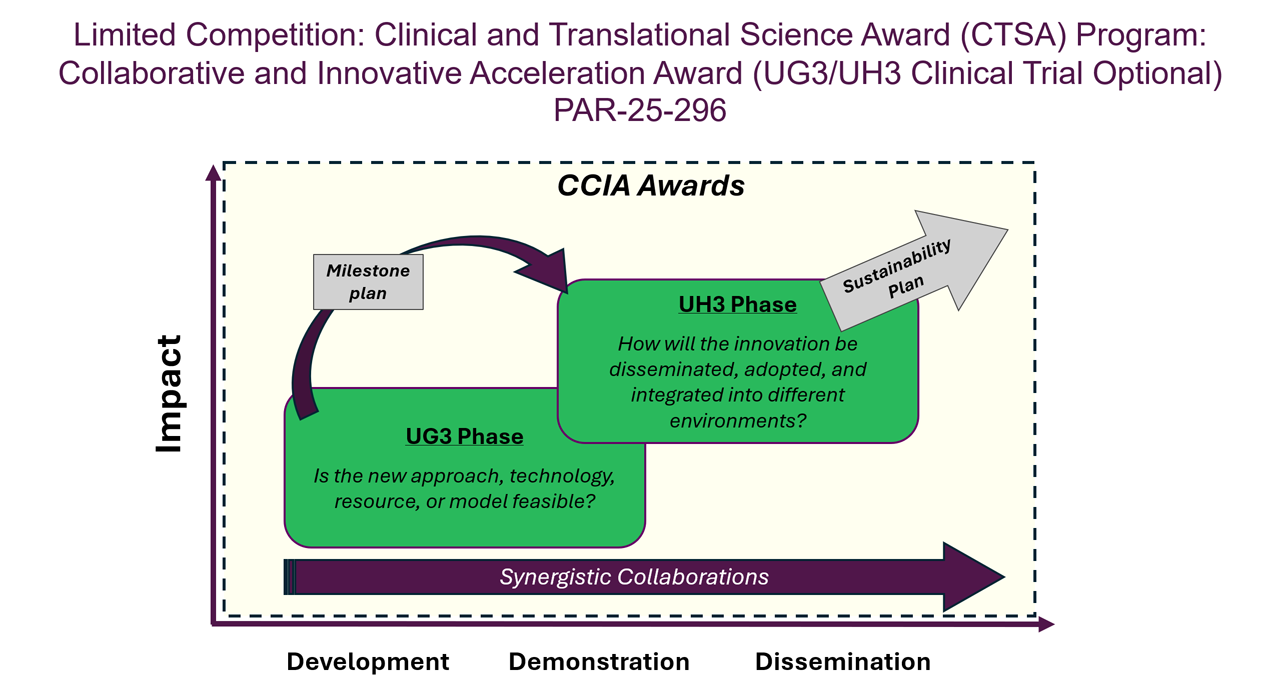
The NCATS CTSA Program supports team-based research to develop, demonstrate and disseminate innovations that can accelerate the translational research process to turn laboratory, clinic and community observations into interventions and benefit public health more quickly. The Collaborative and Innovative Acceleration Award aims to provide support to investigators to tackle a translational science problem no one organization can solve alone. The award supports synergistic activities that accelerate the translational research process through collaboration and innovation.
The notice of funding opportunity invites applications to develop, demonstrate and disseminate innovative solutions to transform the field of translational science by addressing the inefficiencies that are common across diseases and bringing more interventions to all people more quickly through collaborative science among the CTSA Program institutions; NIH institutes, centers, and offices; and/or external stakeholders.
CTSA Program Collaborative Innovation Awards Funding Opportunity
For more information, please contact CTSA Collaboration Innovation and see Application Information for CTSA Program Collaborative and Innovation Acceleration Awards for a list of FAQs.
Funded Awards
CTSA Program Collaborative Innovation Awards (CCIA)
The funded projects reflect the CTSA Program goals and cover a broad spectrum of translational science, ranging from diagnostics and clinical trial design to patient-reported outcomes and community engagement.
- 2024 CCIA project
- 2023 CCIA project
- 2022 CCIA projects
- 2021 CCIA projects
- 2020 CCIA projects
- 2019 CCIA projects
Find earlier CCIA Projects (2016–2018) in NIH RePORTER. Use the filters to sort projects by fiscal year, organization and other details.
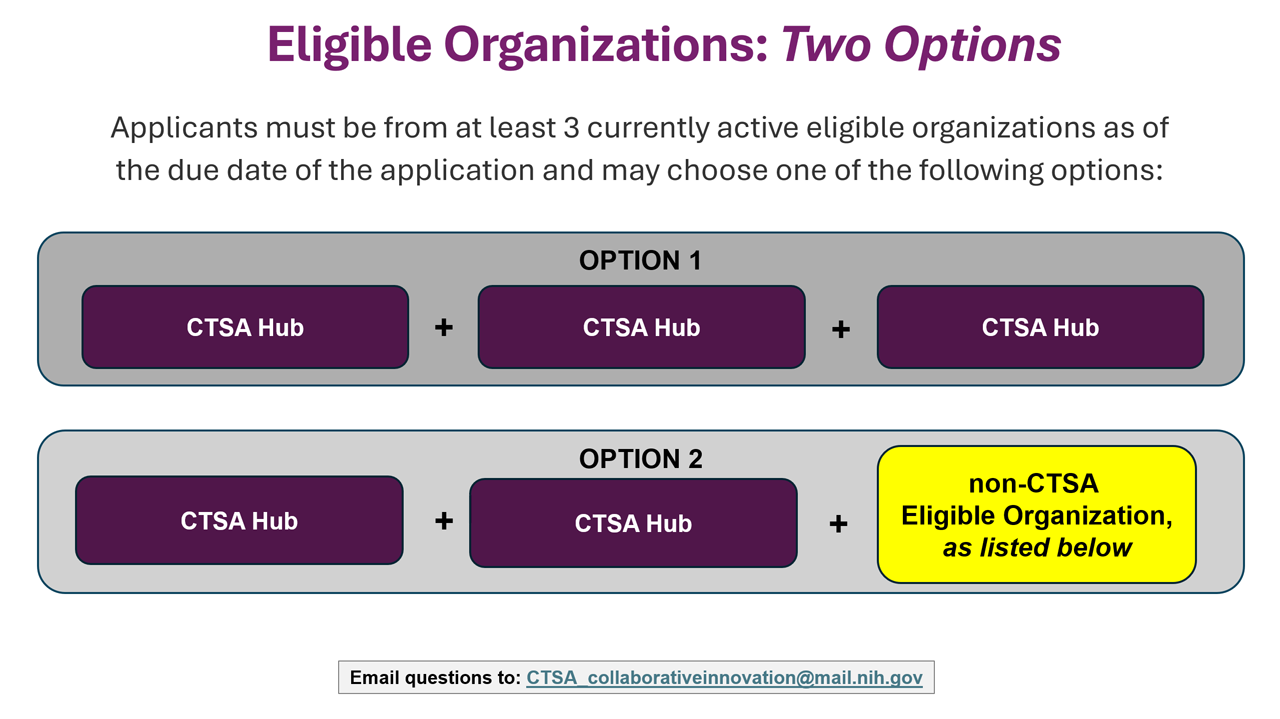
Eligible Organizations
NCATS
- Rare Diseases Clinical Research Network Centers (award recipients funded under RFA-TR-18-020, RFA-TR-18-021 and subsequent reissues)
U.S. Food and Drug Administration
- Centers of Excellence in Regulatory Science and Innovation (award recipients funded under RFA-FD-23-004 and subsequent reissues)
NIAMS
- Core Centers for Clinical Research (award recipients funded under RFA-AR-24-003, RFA-AR-22-002 and subsequent reissues)
- Centers of Research Translation (award recipients funded under RFA-AR-22-001 and subsequent reissues)
NIDCR
- National Dental Practice-Based Research Network (award recipients funded under RFA-DE-19-001, RFA-DE-19-002, RFA-DE-19-006, PAR-20-306 and subsequent reissues)
- Practice-Based Research Integrating Multidisciplinary Experiences in Dental Schools (PRIMED) (award recipients funded under RFA-DE-23-012 and subsequent reissues)
- FaceBase (award recipients funded under PAR-23-237 and subsequent reissues)
NIGMS
- IDeA Clinical & Translational Research Development (CTR-D) (award recipients funded under PAR-23-257 and subsequent reissues)
- IDeA Clinical & Translational Research Network (CTR-N) (award recipients funded under PAR-23-241, PAR-20-175, PAR-18-265 and subsequent reissues)
ODP
- Tobacco Centers of Regulatory Science (TCORS) for Research Relevant to the Family Smoking Prevention and Tobacco Control Act (award recipients funded under RFA-OD-22-004 and subsequent reissues)
- Center for Coordination of Analysis, Science, Enhancement, and Logistics (CASEL) in Tobacco Regulatory Science (award recipients funded under RFA-OD-22-003 and subsequent reissues)
- Center for Rapid Surveillance of Tobacco (CRST) to Assess Changes in Use Behaviors, Product Marketing, and the Marketplace (award recipients funded under RFA-OD-22-002 and subsequent reissues)
ORWH
- Specialized Centers of Research Excellence (SCORE) on Sex Differences (award recipients funded under RFA-OD-22-014, RFA-OD-19-013 and subsequent reissues).