NIH Awards $35.5 Million to Use Tiny, Bioengineered Organ Models to Improve Clinical Trials’ Development and Design
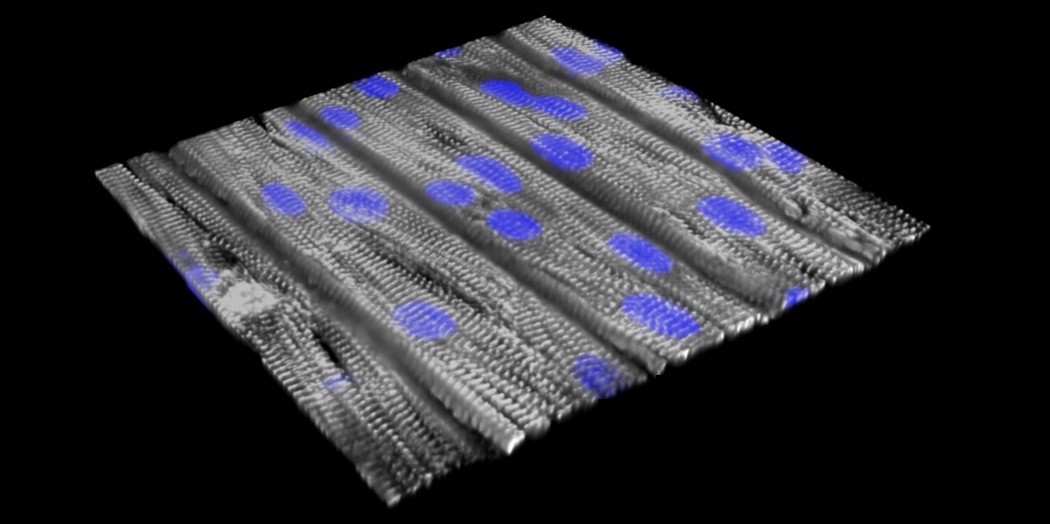
A tissue model of catecholaminergic polymorphic ventricular tachycardia (CPVT), a leading cause of sudden death from cardiac arrest in children and young adults, on a tissue chip. Scientists from Boston Children’s Hospital and Harvard University reprogrammed blood cells from a patient with a gene mutation linked to most cases of CPVT and prompted these cells to become stem cells. These in turn were made into cardiomyocytes (heart muscle cells) carrying CPVT mutations, which were seeded onto an engineered surface. The cells, shown in purple, lined up in a direction similar to how heart muscle is organized and beat together. (Sung Jin Park/Boston Children’s Hospital and Donghui Zhang/Harvard SEAS)
September 29, 2020
Clinical Trials on a Chip researchers plan to build and test common and rare disease models to help improve the clinical trial process.
Approximately 85% of late-stage clinical trials of candidate drugs fail because of drug safety problems or ineffectiveness, despite promising preclinical test results. To help improve the design and implementation of clinical trials, the National Institutes of Health has awarded 10 grants to support researchers’ efforts in using tiny, bioengineered models of human tissues and organ systems to study diseases and test drugs. One major goal of the funded projects is to develop ways to better predict which patients are most likely to benefit from an investigational therapy prior to initiating clinical trials.
The awards total more than $6.9 million in the first year, and approximately $35.5 million over five years, pending available funds. They are administered through a new program, Clinical Trials on a Chip, which is led by NIH’s National Center for Advancing Translational Sciences (NCATS) in conjunction with several other NIH Institutes and Centers, including the National Cancer Institute, the National Institute of Child Health and Human Development, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Tissue chips, or organs-on-chips, are 3-D platforms engineered to support living human tissues and cells and mimic complex biological functions of organs and systems. Tissue chips are currently being developed for drug safety and toxicity testing and disease modeling research, including on the International Space Station. Clinical Trials on a Chip is one of several initiatives that are a part of the NCATS-led Tissue Chip for Drug Screening program, which was started in 2012 to address the major gaps in the drug development process.
The projects are organized into two phases. In the first phase, the grantees will develop tissue chip models of disease. They also will develop biological indicators from the chips that correlate with clinical condition and enable scientists to determine the progression of a disease and effectiveness of a therapy. In the second phase, the researchers will evaluate the usefulness of the disease models by testing candidate therapies and, in some cases, collaborating with pharmaceutical and biotech companies to compare patient results with that of the corresponding patient chips.
Many of these therapies will be tested in tissue chips in parallel to patients in clinical trials. In one project, for example, scientists plan to develop a tissue chip model of two types of muscular dystrophy, a muscle-wasting disease, to study the effects of a promising drug candidate already being tested in patients. Another team of researchers plans to develop a bone marrow model to determine which patients with treatment-resistant prostate cancer that has spread to the bone might benefit from a new therapy that also is being tested in patients.
The new grantees bring expertise in clinical trial design, disease biology, engineering, pharmacology, computational biology and more. They will study a range of common and rare diseases, such as prostate cancer, pediatric disorders, kidney disease, heart disease, fatty liver disease and a disease that causes premature aging as well as premature birth.
“Our hope is to eventually impact the fundamental way we do clinical trials,” said Danilo Tagle, Ph.D., NCATS associate director for special initiatives, who oversees the program, along with scientific program manager Passley Hargrove-Grimes, Ph.D. “We want to see if tissue chips can be a useful platform in which to help researchers preempt some potential challenges with trials. The teams have to make sure the models are valid and show that data from tissue chips accurately reflect results in people. If tissue chip data can predict which patient populations might benefit the most from investigational drugs, this information could potentially improve clinical trial success rates.”
The 2020 awardees are:
Boston Children’s Hospital
William Pu, M.D., and Kevin Kit Parker, Ph.D. (Harvard University’s Wyss Institute for Biologically Inspired Engineering)
Tissue Chips for Precision Treatment of Catecholaminergic Polymorphic Ventricular Tachycardia
Brigham and Women’s Hospital, Boston
Yu-Shrike Zhang, Ph.D.
Clinical Trials on a Premature Vascular Aging-on-a-Chip Model
Cedars-Sinai, Los Angeles
Clive Niels Svendsen, Ph.D.
A Microphysiological Multicellular Organ-on-Chip to Inform Clinical Trials in FTD/ALS
Columbia University, New York City (two-year award)
Angela M. Christiano, Ph.D.
Clinical Trials in a Dish Using a Personalized Multi-Tissue Platform for Atopic Dermatitis
Johns Hopkins University, Baltimore
Deok-Ho Kim, Ph.D., and David Alan Kass, M.D.
Engineering Clinical Trials on a Chip for Dystrophin-Deficient Muscular Dystrophy
Texas A&M University/Texas Engineering Experiment Station, College Station
Arum Han, Ph.D., and Ramkumar Menon, Ph.D. (University of Texas)
Developing Extracellular Vesicle-Based Therapeutics Against Pre-Term Birth Through the Use of Maternal-Fetal Interface on a Chip
University of Pittsburgh
D. Lansing Taylor, Ph.D., Jaideep Behari, M.D., Ph.D., and Alejandro Soto-Gutierrez, M.D., Ph.D.
A Vascularized Patient-Derived iPSC Liver Acinus Microphysiological System as an Innovative Precision Medicine Platform for Optimizing Clinical Trial Design for Nonalcoholic Fatty Liver Disease
University of Rochester
Hani A. Awad, Ph.D., James L. McGrath, Ph.D., and Benjamin L. Miller, Ph.D.
A Microphysiological System of Tendon Inflammation and Fibrosis for Drug Screening and Efficacy Testing
University of Washington, Seattle
Jonathan Himmelfarb, M.D., and Matthias Kretzler, M.D. (University of Michigan)
Safety and Efficacy of Human Clinical Trials Using Kidney-on-a-Chip Microphysiological Systems
University of Wisconsin, Madison
David J. Beebe, Ph.D. and Joshua Michael Lang, M.D.
Mechanisms of Microenvironment Mediated Resistance to Cancer Cell Surface Targeted Therapeutics
Media Contact: info@ncats.nih.gov
About the National Center for Advancing Translational Sciences (NCATS): NCATS conducts and supports research on the science and operation of translation — the process by which interventions to improve health are developed and implemented — to allow more treatments to get to more patients more quickly. For more information about NCATS, visit https://ncats.nih.gov.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit https://www.nih.gov.
NIH…Turning Discovery Into Health®


