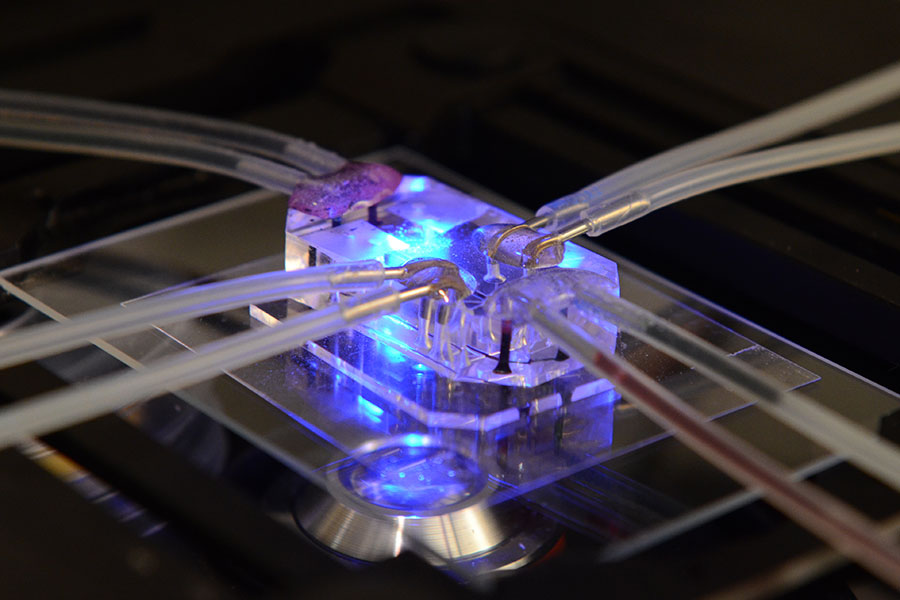
Tissue Chip Projects & Initiatives
The Tissue Chip for Drug Screening program aims to develop bioengineered devices to improve the process of predicting whether drugs will be safe or toxic in humans. Learn more about the projects and initiatives.
About Tissue Chip Projects & Initiatives
During the program’s inception, it has focused on developing physiologically relevant models for toxicity testing. The current focus of the program is on disease modeling and efficacy testing.
- Translational Centers for Microphysiological Systems
- Tissue Chip Development
- Tissue Chip Testing Centers
- Tissue Chips in Space
- Tissue Chips for Disease Modeling and Efficacy Testing
- Tissue Chips for Pain, Opioid Addiction and Overdose (NIH HEAL Initiative project)
- Tissue Chips for Modeling the Immune System
- Clinical Trials on a Chip
- Tissue Chips for Studying COVID-19
Translational Centers for Microphysiological Systems
We have supported the development and use of tissue chips in testing drug candidates for safety and efficacy, modeling diseases, designing clinical trials, and exploring applications for precision medicine. Now, we have established four Translational Centers for Microphysiological Systems (TraCe MPS) to support the widespread adoption and use of tissue chip technology, especially in drug discovery and development. The TraCe MPS program, which is in partnership with the U.S. Food and Drug Administration (FDA), will seek to qualify tissue chips with a defined context of use as FDA-designated drug development tools.
Texas A&M University, College Station and The University of Texas Medical Branch, Galveston
A Translational Center for Microphysiological Systems-Based Drug Development Tools for Pregnancy and Women's Health
Principal Investigators: Ramkumar Menon, Arum Han, Ivan Rusyn
Grant Number: 1U2CTR004868-01
University of Pittsburgh
Qualification of Patient-Derived Biomimetic Liver MPS as Drug Discovery Tools for Drug Metabolism, Toxicity, Drug Efficacy Testing and Clinical Trial Cohort Selection
Principal Investigators: D. Lansing Taylor, Mark T. Miedel, Mark E. Schurdak, Alejandro Soto-Gutierrez, Lawrence A. Vernetti
Grant Number: 1U2CTR004863-01
University of Rochester
A Translational Center for Barrier MPS
Principal Investigators: James L. McGrath, Joan E. Adamo, Hani A. Awad, Benjamin L. Miller, George A. Truskey
Grant Number: 1U2CAG088071-01
University of Washington, Seattle and the Icahn School of Medicine at Mount Sinai
Translational Center for Kidney Microphysiological Systems to Improve Drug Safety and Efficacy
Principal Investigators: Jonathan Himmelfarb, Benjamin Solomon Freedman, Edward J. Kelly
Grant Number: 1U2CTR004867-01
Tissue Chip Development
The first two-year funding phase of the Tissue Chip for Drug Screening program (2012–2014) supported the development of 3-D cellular microsystems designed to represent a number of human organ systems. Renewable cell sources and bioengineered microsystems that successfully demonstrated physiological function moved into the next three-year phase (2015–2017) to further refine the technology and begin organ chip integration, with the first five years of the program drawing to a close in July 2017. Projects that explored the use of stem and progenitor cells to differentiate into multiple cell types that represent the cellular architecture within the organ were also awarded through this initiative. Learn more about Tissue Chip Development.
Tissue Chip Testing Centers
Tissue Chip Testing Centers were based at independent institutions and provided a way to test and validate tissue chip platforms developed through the program. These efforts helped validate tissue chip technology and promoted the adoption of this technology by the broader research community. Learn more about Tissue Chip Testing Centers.
Tissue Chips in Space
We partnered with the International Space Station U.S. National Laboratory (ISS National Lab) on its Tissue Chips in Space initiative. Through this initiative, NCATS and the ISS National Lab collaborated to refine tissue- and organ-on-chip platforms for on-flight experiments at the space station so that scientists can better understand diseases and translate those findings to improve human health on Earth. Learn more about Tissue Chips in Space.
Tissue Chips for Disease Modeling and Efficacy Testing
The Tissue Chips for Disease Modeling initiative supported further development of tissue chip models of human disease that mimic the pathology in major human organs and tissues. The goals were to (1) support studies to develop in vitro disease models using primary tissue or induced pluripotent stem cell (iPSC)-derived patient cell sources on tissue/organ-on-chip platforms, (2) determine disease relevance of these models by preliminary testing of key experimental features and (3) test the effectiveness of candidate drugs.
Tissue Chips for Pain, Opioid Addiction and Overdose (NIH HEAL Initiative project)
With funding from the Helping to End Addiction Long-termSM Initiative, or NIH HEAL InitiativeSM, we awarded five grants for research teams to create and test tissue chips to understand the mechanisms or effects of nociception (the sensory system’s response to harmful stimuli, including pain-relevant signaling), addiction and opioid use disorder. Learn more about tissue chips for pain, opioid addiction and overdose and view the awards.
Tissue Chips for Modeling the Immune System
Gaining a better understanding of the immune system and its interactions with other physiological systems is a critical research need. To address this need, we and the National Institute of Biomedical Imaging and Bioengineering funded four research projects focused on developing tissue chips that model components of the human immune system.
Clinical Trials on a Chip
For people afflicted with difficult-to-treat, life-threatening diseases, clinical trials are an important strategy for finding effective new and repurposed therapies. Unfortunately, around 85 percent of late-stage clinical trials of investigational drugs fail because of safety problems or ineffectiveness despite promising preclinical test results in conventional models. To improve the rate of success of new therapeutics in drug development, the Clinical Trials on a Chip program, led by NCATS in conjunction with several other NIH institutes and centers, supports researchers’ efforts to create microphysiological, bioengineered models of human tissues and organ systems to inform clinical trial design, support the planning and execution of clinical trials, assist in patient stratification, help identify reliable clinical trial endpoints, and ultimately develop tools for more informative and efficient clinical trials.
Tissue Chips for Studying COVID-19
With support from the Coronavirus Aid, Relief, and Economic Security (CARES) Act, we were able to rapidly award additional funding to tissue chip investigators so that they could pivot their work to evaluate properties of SARS-CoV-2, the virus that causes COVID-19; identify and test potential treatments and therapeutics for COVID-19; and examine the mechanisms of COVID-19 disease pathology.