3-D Tissue Bioprinting: An Emerging Path to Better Drug Development
Translational Science Highlight
- NCATS is addressing a new area of opportunity to better predict how potential new drugs will affect humans. Center scientists are developing 3-D tissue models that more closely mimic the complexity of tissues in the human body in a reproducible, automated and scalable manner using bioprinting techniques. These innovative, human-like tissue models are used for compound testing and could accelerate drug development for treatments of both rare and common diseases.
Current methods of developing and delivering new drugs to patients take decades, cost billions of dollars and are unsuccessful about 95 percent of the time. The vast majority of potential new drugs fail in clinical trials because they are found to be ineffective or are unexpectedly toxic for patients, despite promising early results during preclinical testing.
Traditional drug development involves analyzing the effects of potential drugs in 2-D laboratory-grown cells that have indicators of disease — so-called in vitro assays — and in laboratory animal models that have been developed with a disease either the same as or similar to a disease in humans.
As one effort to improve the predictive success of preclinical drug development, NCATS launched the 3‑D Tissue Bioprinting program. NCATS researchers are using the techniques of 3-D bioprinting to combine living cells with scaffolding materials, to create testing platforms of laboratory-grown human tissues that closely mimic natural tissues in human organs. These innovative tissue models could help scientists better predict the way patients will respond to potential new therapies.
Working in Three Dimensions

Kristy Derr with a 3-D printer.
“The way we do compound testing for drug discovery now is using cells that are grown in 2-D on plastic surfaces,” said Marc Ferrer, Ph.D., director of the NCATS 3-D Tissue Bioprinting program. “It’s easy to grow cells in 2-D, but that’s not how cells grow in the human body. By recreating the body’s 3-D tissue structure through 3-D bioprinting, we can grow cells in the same way as they are found in the body. The hope is that the cells will respond to potential drugs in the same way that they do in a patient.”
The 3-D Tissue Bioprinting program builds on internal expertise at NCATS in assay development for drug screening, automation technologies, stem cell biology, tissue engineering and advanced imaging techniques. Living cells and scaffolding, or support, materials can now be combined into complex 3-D functional tissues produced on multi-well plates (tissue-in-a-well), which are used in preclinical drug testing. These tissues are created using either induced pluripotent stem cells (iPSCs) or primary cells taken directly from living tissue, such as skin.
“Bioprinting tissues is an art,” Ferrer said. “You have to mix human cells with Jell-O–like materials called bioinks that enable the bioprinting of the cells in a specific geometry that resembles human tissues. The mixture of cells and bioinks can’t be too thick or too thin — it has to be just the right consistency so that the cells can be dispensed through a needle, stay alive and form the shape of the tissue.”
Achieving Reproducibility
The primary focus of the 3-D Tissue Bioprinting program is to develop “disease-relevant tissue models” that can be tested against various compounds to learn with greater accuracy how potential new treatments might affect the body. A challenge in using 3-D tissue models as a drug screening platform is producing tissues that look the same, function consistently and are reproducible from assay to assay in a multi-well plate format. This consistency helps ensure that screening results are reliable. With the use of 3-D bioprinters, NCATS can begin to industrialize production of 3-D tissues in an efficient, reproducible and scalable manner.
Sam Michael, NCATS’ chief information officer and director of automation and compound management, said the challenges of refining the technology to make it reliably reproducible are daunting.
“The printer is the simplest part of the process,” Michael said. “We can print tissues in about 30 minutes, as opposed to it taking months to grow them in plates.”
The challenge is making the bioprinter configurable for different types of tissue.
“We need to be able to use a variety of dispensing techniques,” Michael explained. “It’s so complex, and points of failure are so many, that I’m amazed any of this works. You have to be resilient, because most of what we do fails. It’s like a sliver of success versus an ocean of failure.”
Collaborating with Disease Experts
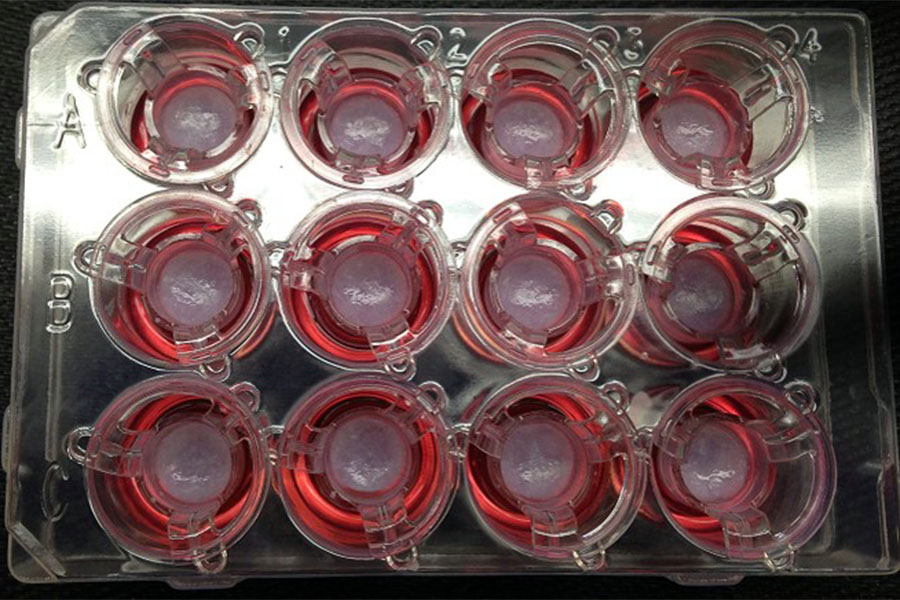
Pictures of 3-D bioprinted tissues in a 12-well transwell plate showing reproducible tissue shape from well to well.
Two initial areas of investigation for 3-D bioprinting involve age-related macular degeneration (AMD) — the leading cause of irreversible blindness — and skin diseases.
For the AMD project, collaborators from NCATS and the National Eye Institute (NEI) are creating 3-D bioprinted retinal tissue to develop in vitro disease models. The goal is to study patient-specific disease processes, set up high-throughput drug screening tests and develop cell-based therapies for retinal degenerative disease.
Initial work is focused on the retinal pigment epithelium (RPE), a layer of cells located in the back of the eye. Researchers suspect that abnormal changes in the RPE can lead to degenerative eye diseases such as AMD. The NCATS/NEI collaboration combines iPSCs produced from patients’ cells and high-throughput screening assays to identify novel compounds that could potentially reverse the effects of these devastating diseases.
NCATS is also collaborating with Rockefeller University and Columbia University in New York City to develop 3-D printed skin tissue that can be used to investigate possible therapies for diseases such as cancer and psoriasis. The skin is the largest organ of the human body. It is highly complex and provides protection from the environment, microbes, parasites, heat, ultraviolet rays and water loss. 3‑D printing of skin promises to be an invaluable way to accurately test potential disease therapies.
Applications for the Future
Bioprinted tissues and their associated technologies have promise for civilian and military research applications, such as pandemic diseases, biodefense and national security. For example, cell and tissue replacement for injured soldiers and development of novel medicines for field use — such as antibiotics and drugs for mental illness, trauma and surgery — are areas of huge potential.
“We’re working to develop better assays for drug discovery, so that we can make the process more efficient,” Ferrer said. “The closer the testing platforms are to mimicking humans, the better we will be at discovering new drugs for the patients who need them.”
Posted September 2018


