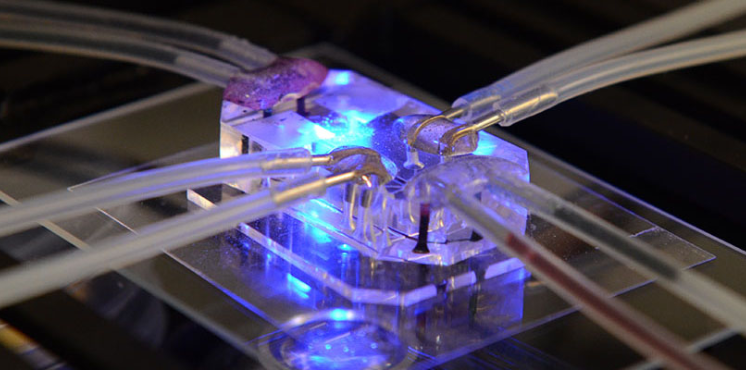
Tissue Chip for Drug Screening
The Tissue Chip for Drug Screening program aims to develop bioengineered devices to improve the process of predicting whether drugs will be safe or toxic in humans.
Tissue Chip Overview
Contacts
Passley Hargrove-Grimes, Ph.D.
Dmitriy V. Krepkiy, Ph.D.
View the 3-D Tissue Models fact sheet (PDF - 222KB)

Many promising medications have failed to be safe and effective in human clinical trials despite promising preclinical studies. We are addressing this problem through the Tissue Chip for Drug Screening program. We coordinate with other NIH institutes and centers and the U.S. Food and Drug Administration (FDA).
Tissue chips are built from human cells. Also called organs-on-chips, they mimic the structure and function of our heart, kidneys, lungs and other organ systems. Scientists are developing and using tissue chips to test the potential effects of drugs on those tissues in a faster and more effective way than current methods. Learn more about these projects.
With flexible funding from the Cures Acceleration Network, we focus on developing high-need cures and reducing major barriers between research discovery and clinical trials. Our Tissue Chip for Drug Screening program aims to speed the translation of basic discoveries into the clinic. By creating an integrated human body-on-a-chip, researchers will be able to test the possible effects of a drug or other substance across the entire body before testing in people.
Because they use human cells, tissue chips are also useful research tools to study human diseases and conditions when animal models do not mirror the pathology or are unavailable. They support NIH’s position that non-animal model approaches can reduce the need for animals in research but will require further improvement to completely replace them.
Tissue Chip News

Tissue Chips in Space 2.0 Will Reveal Age-Related Disease Mechanisms and Possible Therapies
February 17, 2026 - NCATS News
- Tissue Chip for Drug Screening
NIH selected researchers to design microphysiological technologies that mimic human organ systems for age-related disease experiments at the International Space Station National Laboratory.
Read ArticleTissue Chips in Space 2.0 Will Reveal Age-Related Disease Mechanisms and Possible Therapies
February 17, 2026 - NCATS News
- Tissue Chip for Drug Screening
NIH selected researchers to design microphysiological technologies that mimic human organ systems for age-related disease experiments at the International Space Station National Laboratory. These studies will provide key preclinical data and may inform clinical trials for aging-related disease mechanisms and potential therapies.
Physiological Human In Vitro Models to Study and Evaluate Tumor Immunology
February 9, 2026 - Media Coverage
- Tissue Chip for Drug Screening
A Future Beyond Animal Testing: Why ORIVA Matters and How Computational Models Bridge the Gap
November 14, 2025 - Media Coverage
- Tissue Chip for Drug Screening
Why Tissue Chips Matter
Watch the Tissue Chip for Drug Screening video (video length: 3:49) to learn more about the program.
Related Research

Stem Cell Translation Laboratory
Our experts develop methods and standards to use stem cell technology (using cells derived from skin
or blood) to advance treatment approaches.
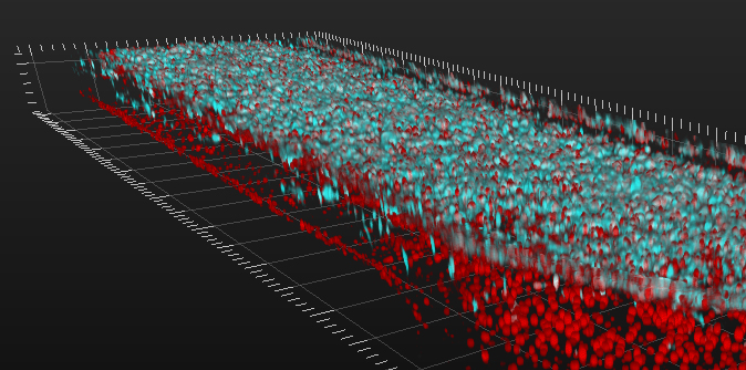
3-D Tissue Bioprinting
Our scientists are creating and using 3-D printing techniques to make tissue models that closely resemble the complex structure and organization of our cells.
Matrix Combination Screening
Our experts use matrix combination screening technology to quickly identify promising drug combinations with the most potential to help patients.