Appendices
Appendix A: Additional Details on NCATS Organization
Statutory Authority
Establishment of NCATS
NCATS was created on December 23, 2011, by the Consolidated Appropriations Act, 2012 (P.L. 112-74), which amended the Public Health Service (PHS) Act by including authorization language for NCATS. The 21st Century Cures Act (P.L. 114-255), which became law on December 13, 2016, subsequently modified NCATS’ authorization language.
The current PHS Act authorization language for NCATS outlines the purpose of NCATS, specifies the phases of clinical trials that may be supported, mandates the NCATS biennial report, and details the previously existing NIH programs that were moved to NCATS, such as the Cures Acceleration Network (CAN).
Cures Acceleration Network
CAN was established within NIH on March 23, 2010, by the Patient Protection and Affordable Care Act (P.L. 111148), but it was not appropriated any funds. Several interested parties wrote a letter to Congress (PDF — 35KB) on May 14, 2010, asking Congress to provide funding for CAN.
On December 23, 2011, the Consolidated Appropriations Act, 2012, appropriated $10 million for CAN and moved CAN to NCATS.
The purpose of CAN is to award grants and contracts to eligible entities to accelerate the development of highneed cures, including through the development of medical products and behavioral therapies.
To learn more about NCATS, visit our website:
Appendix B: Accomplishments From the 2016 NCATS Strategic Plan
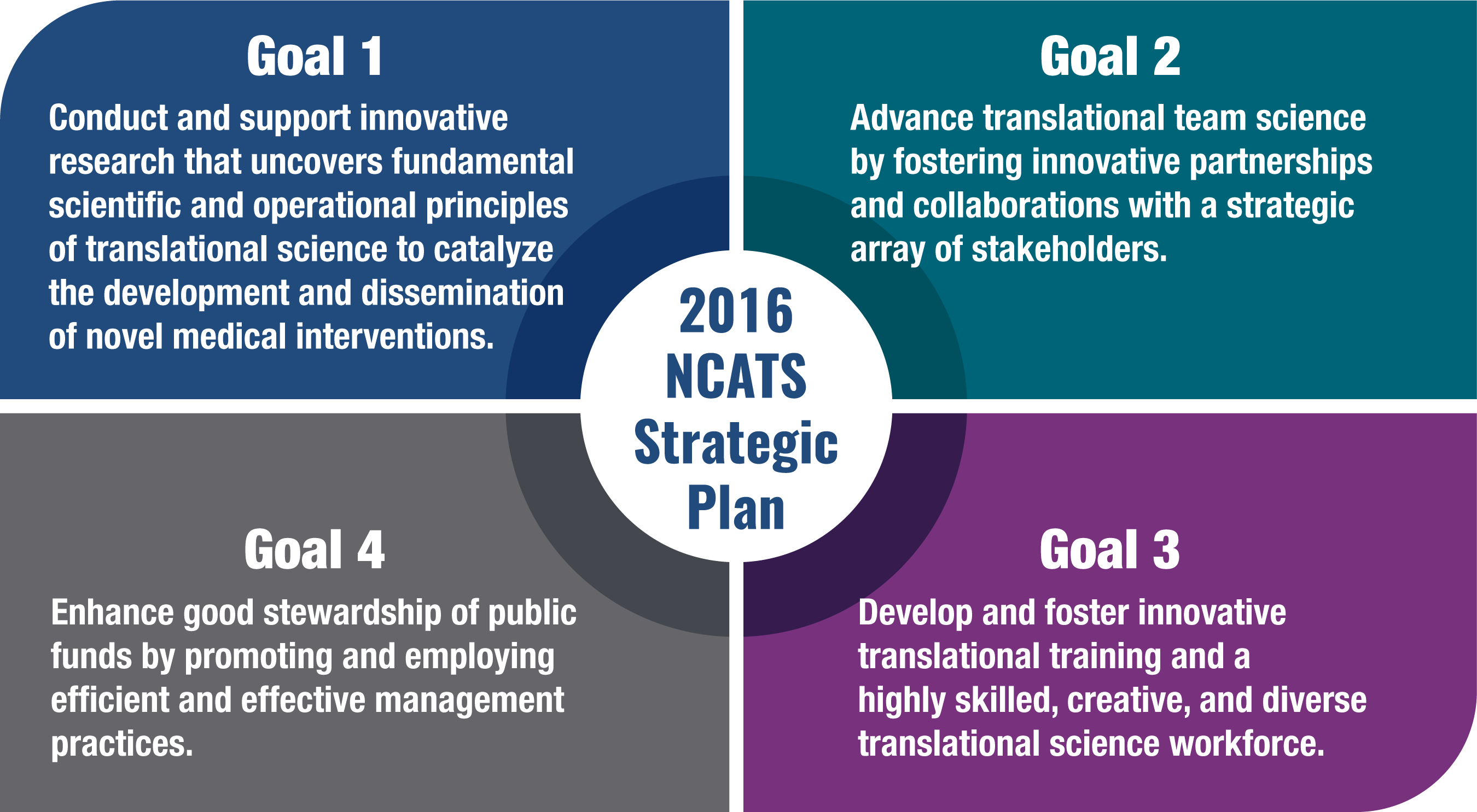
The 2016 NCATS Strategic Plan was released five years after NCATS was established and set a roadmap for the center’s early activities. The release of the NCATS Strategic Plan for 2025-2030 offers an opportunity to reflect on and recognize the many ways in which the 2016 NCATS Strategic Plan goals were accomplished and how NCATS led the way in advancing translation science (Figure B-1).
NCATS has made progress in meeting the goals of the 2016 NCATS Strategic Plan in several key areas, highlighted below. Find more details and impact examples from these activities in the NCATS Congressional Justifications (found on our Budget web page), NCATS biennial reports, NCATS Areas of Impact, and NCATS News & Events.
Innovation and Impacts in Clinical Trials (Goals 1, 2, and 3)
NCATS has played a leading role as an innovator in the conduct and efficiency of clinical trials. The Institutional Review Board (IRB) and recruitment and operations processes have been revitalized through the Clinical and Translational Science Awards (CTSA) Streamlined, Multisite, Accelerated Resources for Trials IRB (SMART IRB) Platform and the Trial Innovation Network. Leveraging the scope and capabilities of the CTSA Program network enabled NCATS to rapidly respond to the COVID-19 pandemic through the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) trials (ACTIV-1 and ACTIV-6). The Rare Diseases Clinical Research Network (RDCRN) has built a collaborative rare diseases research network that has resulted in 12 U.S. Food and Drug Administration (FDA)–approved treatments for 11 of the diseases studied in this network.
Rare Disease Advancements (Goals 2 and 4)
NCATS is a home for rare disease advancements that too often get stuck in the translational pipeline. The center raises awareness of the economic burden of rare diseases through activities like the IDeaS (Impact of Rare Diseases on Patients and Healthcare Systems) study. NCATS also disseminates information and resources through the Genetic and Rare Diseases (GARD) Information Center and through partnerships established in programs like the Accelerating Medicine Partnership® (AMP®) Bespoke Gene Therapy Consortium (BGTC) and Platform Vector Gene Therapy (PaVe-GT). These efforts increase the potential to address many diseases at a time.
Expansion of Training Opportunities in Translational Science (Goal 3)
NCATS provides robust training experiences for new translational scientists through our numerous internal fellowship opportunities and various awards to our external collaborators. Additionally, the NCATS Office of Strategic Alliances provides training in the business areas surrounding translational science. Activities include internal lunch-and-learn programs that are open to all NCATS staff and trainees and the internal NCATS Advancing Innovation through Mentorship (AIM) program, which is fashioned after the National Science Foundation iCorps program.
Harnessing the Power of Data Science (Goals 1, 2, and 4)
At NCATS, we have invested in new ways to connect, access, and learn from large and complex data sets. We also create and use data tools and methods in new ways to speed translational research. Activities span the translational pipeline, from using platforms like Biomedical Data Translator and OpenData Portal to identify promising therapeutic candidates and avenues to harnessing the power of clinical data to address urgent public health needs through the National Clinical Cohort Collaborative (N3C) and CURE ID.
Developing and Utilizing Human Cell Models (Goals 1 and 2)
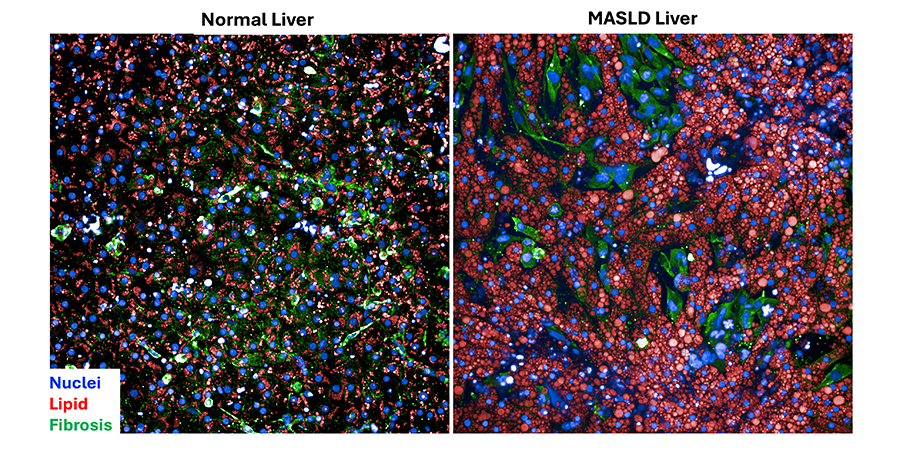
NCATS is providing key advances in innovative systems to mimic diseases and test potential treatments in efficient and validated human cell models. We established key infrastructure, including the Stem Cell Translational Laboratory and 3-D Tissue Bioprinting Program, and developed tools to work with these systems, like Somatic Cell Genome Editing. In particular, our Tissue Chips for Drug Screening Program has led the development of 3-D platforms designed to represent human organ systems and mimic functions of the human body (Figure B-2). Tissue chips have allowed the testing of treatments for many diseases or conditions, including addiction and pain and COVID-19, as well as understanding aging by sending tissue chips into space.
Increasing Awareness of Translational Science as a Discipline (Goals 3 and 4)
One goal of our inaugural strategic plan was to increase awareness of translational science as a discipline. To this end, the NCATS Communications Branch developed and widely disseminated new resources that describe NCATS’ work in crisp, clear, and compelling language. NCATS also developed the NCATS Translational Science Principles, which stem in part from in-depth case studies and build on scholarship identifying core competencies for translational science.
Engaging and Facilitating Novel Partnerships (Goals 2 and 4)
NCATS has led the way in showing how to establish and conduct successful collaborations through such programs as A Specialized Platform for Innovative Research Exploration (ASPIRE) and leadership in NIH Common Fund programs like the Extracellular RNA Communication Program. NCATS also has established a new version of the standard scientific partnership agreement with the innovative cooperative research collaboration agreement template. This and other templates speed the implementation of research agreements. Thanks to NCATS’ facilitation of partnerships, three major exclusive licenses are now in different stages of their life cycles.
Enhancing NCATS’ Internal Processes (Goal 4)
Stewardship of all NCATS activities was instrumental to achieving the goals of the 2016 NCATS Strategic Plan. During this time, NCATS developed a user-friendly internal human resources system — the K2 ProgressivE Enterprise Personnel System and workflow automation application. It is one example of how we enabled an administrative structure to align key operational support and infrastructure in support of the NCATS mission. NCATS has also established a transparent and proactive center-wide operations planning process. To improve the awards management process, the NCATS Division of Extramural Activities (DEA) led the effort to establish Other Transaction Awards as a new business line at the NIH level, which is critical to the conduct of the Cures Acceleration Network. DEA established prize competitions as a new mechanism for funding and developed best practices for engaging NIH institutes and centers as partners, instead of as a service center, as exemplified by the partnership with the National Institute on Drug Abuse for managing the Native Collective Research Effort to Enhance Wellness (N CREW) Program, under The Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®.
Achieving Crucial Regulatory Milestones (Goals 1 and 2)
Advancing treatments through the translational pipeline requires navigating the regulatory process of therapeutic approval. NCATS has engaged in NIH-wide partnerships to develop novel therapies to the point of readiness to conduct clinical trials for regulatory approval, and the FDA has approved 56 Investigational New Drug (IND) clearances based on research involving the NCATS Division of Preclinical Innovation. The preclinical research conducted by NCATS was also key to de-risking the clinical trials leading to the approval of four drugs (Figure B-3).
Appendix C: Strategic Planning Process
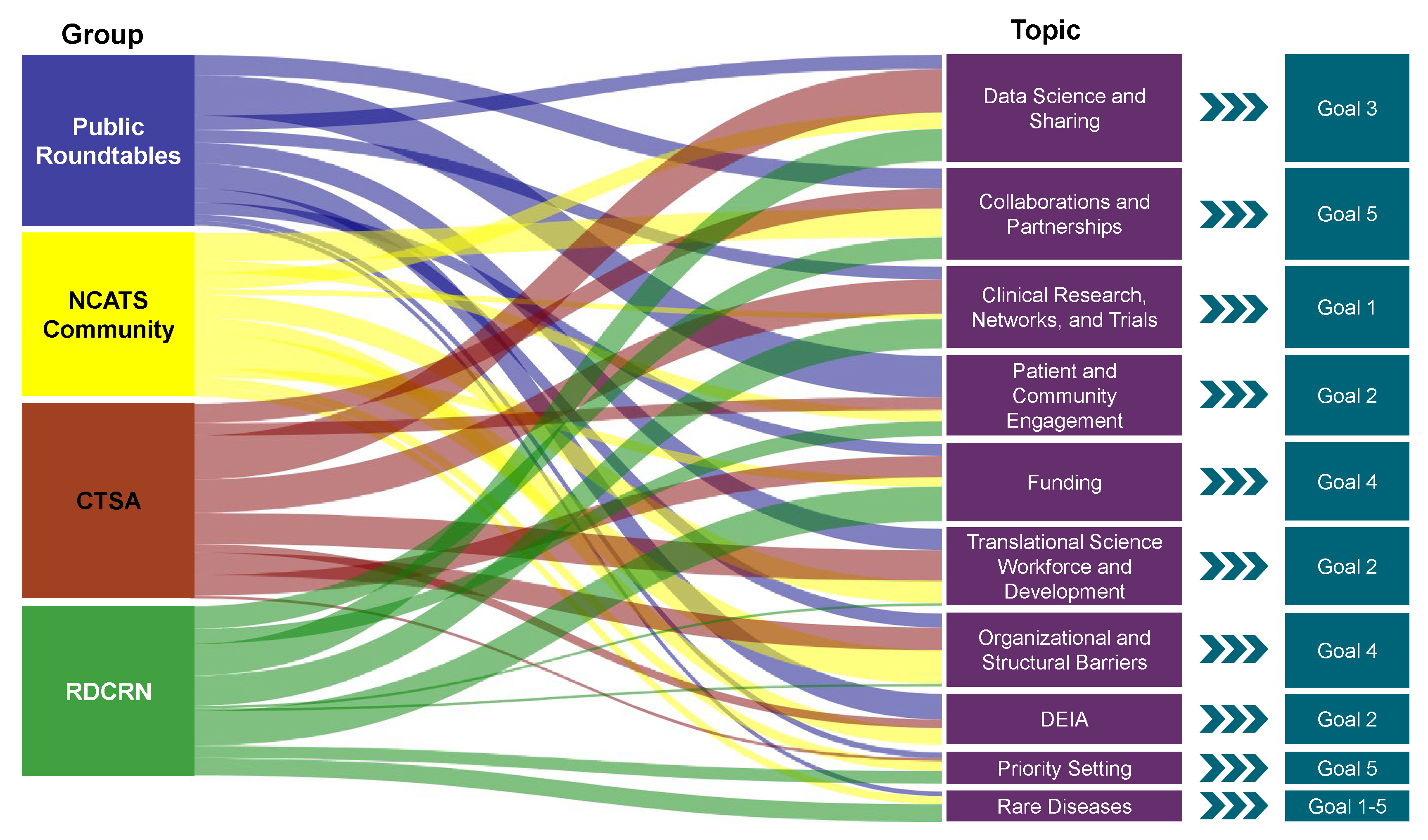
In November 2022, following the appointment of Dr. Joni L. Rutter as the new director, the center began the process of updating the NCATS Strategic Plan that was released in fall 2016. The strategic planning team formed to manage the day-to-day activities of the strategic planning process. The team was based in the NCATS’ Science Policy Branch and included staff from the Office of Policy, Communications and Education and the NCATS Office of the Director. As described below, all parts of the center as well as of the external community have contributed feedback and input throughout the entire process.
The almost two-year-long process encompassed robust community engagement, development of a strategic framework, a request for public and internal input on the framework, and drafting of the plan, while incorporating feedback at each stage (Figure C-1).
Community Engagement
From January 2023 to August 2023, Director Rutter and the strategic planning team participated in over 44 meetings with more than 1,150 individuals. These discussions gathered input on the future of NCATS activities, unmet needs in translation, and opportunities to make progress toward bringing new health solutions to people faster:
- We held 19 virtual discussions involving more than 350 NCATS staff members.
- We hosted two virtual strategic planning roundtable discussions for the public on May 9 and 10, 2023. During these discussions, Dr. Rutter gave an overview of her vision for NCATS and information on the strategic plan. Participants then met in breakout groups to offer their perspectives on different translational science topics. All breakout sessions answered the same four broad, open-ended questions to solicit input.
- Dr. Rutter presented the strategic plan at 24 virtual meetings of the CTSA Program committees and at two meetings with researchers and patient advocacy groups who are part of the RDCRN. These conversations included questions tailored for each particular group. Dr. Rutter also took other opportunities through public speaking engagements to discuss and engage individuals and groups on the strategic plan.
- The NCATS Advisory Council discussed and gave input on the strategic plan at their meetings in May 2023, September 2023, January 2024, and May 2024.
We routinely promoted the strategic plan through different communications channels and solicited input through a public email address to receive additional comments or questions.
After releasing the strategic plan, we will continue to engage internal and external groups in dialogue about our activities and progress.
Framework Development
Referring to a set of relevant, recurring themes identified from the feedback, we categorized the more than 1,700 comments received across strategic engagements (Figure C-2). Using this semiquantitative analysis of the input received, we developed a draft framework for the NCATS Strategic Plan for 2025-2030. The framework presented five strategic plan goals, a brief narrative context of each goal, and a set of potential themes aligned to the NCATS vision.
Framework Input
In September 2023, we presented a draft framework at the NCATS Advisory Council meeting and published a request for information (RFI) (NOT-TR-23-027) via an NIH Guide Notice. The RFI asked for feedback on the draft goals and plans presented in the framework. We received 51 separate responses.
During the period the RFI was open for comments, Dr. Rutter held three small-group discussions with other NIH institute and center directors. In addition, the strategic planning team presented the draft framework at an NCATS town hall meeting for staff and held five virtual discussions, one for each goal, with NCATS staff.
Document Drafting and Refinement
Following external and internal feedback on the draft framework, the strategic planning team drafted objectives. The goals and objectives were further discussed with NCATS leadership. The full strategic plan draft was published on the NCATS website from May 14 through June 14, 2024. It was presented and discussed at the NCATS Advisory Council in May 2024. We received over 20 written responses on the draft and made edits to the strategic plan as applicable. Many comments were more relevant to the implementation process and have been catalogued for implementation (see Appendix D).
Appendix D: Implementation of the Strategic Plan
The NCATS Strategic Plan for 2025-2030 sets forth a vision with five goals, each with objectives for implementation. By continuously evaluating our progress and adapting our strategies, we are committed to making a significant impact on advancing translational science and improving health outcomes.
As the strategic plan was developed, all divisions and offices provided input at multiple steps to ensure centerwide engagement. Involvement at all levels of NCATS will continue after the NCATS Strategic Plan for 2025-2030 is released, starting with internal center leadership discussions in fall 2024.
Consideration of strategic plan implementation is already underway internally. It will be coordinated across the center through the immediate Office of the NCATS Director. Implementation will include prioritizing activities to pursue, as well as putting in place a strategy to track progress, develop metrics, and keep our communities informed as we evaluate our progress.
To start, the NCATS Strategic Plan for 2025-2030 will be used as a reference point for guiding all programs and initiatives that NCATS implements going forward. For example, internal planning is an NCATS-wide activity that occurs two to four times per year during which new ideas are brainstormed and discussed by NCATS staff and leadership to promote transparency, collaboration, coordination, and priority setting. This includes mapping programs and activities to the relevant strategic plan goals and objectives to ensure alignment. As part of this process, starting in October 2024, staff submitting proposals for activities and programs will identify the relevant strategic plan goal(s) and objective(s). Concept clearances for the NCATS Advisory Council also will incorporate this information.
Discussion of potential metrics of the progress in achieving our strategic plan’s goals and objectives and the impact of our programs and activities is ongoing. We will use a variety of approaches to systematically monitor and collect accomplishments and other indicators of progress. We will continue discussions with our constituents to inform how we think about what is impactful in terms of progress in fulfilling our mission and vision.
We will continue to ensure that our implementation aligns with White House, HHS and NIH priorities and plans. Center-specific activities recently initiated include developing roadmaps for NCATS’ pillars of organizational culture and impact and center-wide data science strategies.

(NCATS)
Appendix E: Acronym List
| ACTIV | Accelerating COVID-19 Therapeutic Interventions and Vaccines |
| AI | artificial intelligence |
| AIM | Advancing Innovation through Mentorship |
| AMP® | Accelerating Medicines Partnership® |
| ASPIRE | A Specialized Platform for Innovative Research Exploration |
| BGTC | Bespoke Gene Therapy Consortium |
| CAB | community advisory board |
| CAN | Cures Acceleration Network |
| CTSA | Clinical and Translational Science Awards |
| DEA | Division of Extramural Activities |
| EHR | electronic health record |
| FAIR | findable, accessible, interoperable, and reusable |
| FDA | U.S. Food and Drug Administration |
| GARD | Genetic and Rare Diseases Information Center |
| IDeaS | Impact of Rare Diseases on Patients and Healthcare Systems |
| IRB | Institutional Review Board |
| MASLD | metabolic dysfunction–associated steatotic liver disease |
| ML | machine learning |
| N3C | National Clinical Cohort Collaborative |
| NCATS | National Center for Advancing Translational Sciences |
| NIH | National Institutes of Health |
| PAG | patient advocacy group |
| PaVe-GT | Platform Vector Gene Therapy |
| PHS | Public Health Service |
| RDCRN | Rare Diseases Clinical Research Network |
| RFI | request for information |
| SMART IRB | Streamlined, Multisite, Accelerated Resources for Trials IRB |
| TIN | Trial Innovation Network |