Our Impact on Clinical Trials
We develop innovative strategies for clinical trials so that new therapies can improve the health of all the people who need them.
Shaping the Future of Clinical Trials
Clinical trials are a necessary step on a treatment’s journey to the medicine cabinet. But the barriers to success in clinical trials are high. A clinical trial’s design and approval can take a long time. Even after completion, a trial may not have enough participants to produce robust results, or the results don’t demonstrate that a treatment will work for everyone who needs it.
NCATS improves clinical trial practices so more safe and effective therapies can be efficiently tested and developed for the people who need them. We work with our partners to develop trial designs for testing multiple drugs and best practices for engaging the community at all stages. These approaches allowed major clinical trials for potential COVID-19 treatments to swiftly offer definitive results.
More innovative clinical trial approaches are on the horizon, especially for rare diseases. We are testing whether biomedical devices that use a patient’s own tissues could speed trial design and execution. We’re also developing a clinical trials approach that tests a single therapy on multiple diseases, as well as an approach for increasing the success of clinical trials for rare diseases.
Impact Stories
Immune Modulator Drugs Improved Survival for People Hospitalized with COVID-19
We coordinated and oversaw the ACTIV-1 Immune Modulators clinical trial, which demonstrated improvement in survival rates.
Efficient Registry Approach Powers Practice-Shaping Infant Heart Surgery Trial

Clinical trial resources from NCATS streamlined a clinical trial study on infants undergoing surgery for congenital heart disease.
Clinical Trial Innovation Activities
Our programs help turn clinical trials into meaningful results.

Clinical and Translational Science Awards (CTSA) Program
The CTSA Program addresses the development and implementation of national standards and best practices for translation, from basic discovery to clinical and community-engaged research.

Rare Diseases Clinical Research Network (RDCRN)
We oversee this NIH-wide grant program that supports medical research on over 200 rare diseases through clinical studies, including collaborations, study enrollment and data sharing.

Clinical Trials on a Chip
The Clinical Trials on a Chip program seeks to improve the success rate of new therapeutics in drug development using tissue chips.
Clinical Trial Innovation News
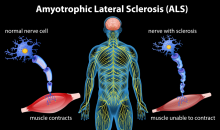
Protein in the Blood Can Help Predict ALS Survival and Decline, Play Role in Developing Treatments
May 13, 2025 - NCATS News
- Our Impact on Clinical Trials
- Our Impact on Rare Diseases
- Rare Diseases Clinical Research Network (RDCRN)
Researchers showed how the level of a protein found in the blood might be used in developing new drugs to treat ALS.
Read ArticleRecommendations and Practical Tools Issued for Conducting Ethical First-in-Human Pig Kidney Xenotransplant Clinical Trials
July 10, 2025 - Grantee/Partner News
- Our Impact on Clinical Trials
How Do Middle-Aged Folks Get Dementia? It Could Be These Proteins
May 14, 2025 - Grantee/Partner News
- Our Impact on Clinical Trials
Protein in the Blood Can Help Predict ALS Survival and Decline, Play Role in Developing Treatments
May 13, 2025 - NCATS News
- Our Impact on Clinical Trials
- Our Impact on Rare Diseases
- Rare Diseases Clinical Research Network (RDCRN)
Researchers showed how the level of a protein found in the blood might be used in developing new drugs to treat ALS.