CTSA Prior Approval Requests Frequently Asked Questions
The questions compiled here represent questions from the CTSA recipient institution community discussed in the CTSA Quality Assurance/Quality Control Group meeting and questions posted in the QA/QC Group Discussion Forum. Responses provided in this document are in accordance with NIH Grants Policy and do not supersede recipient institutional policies.
The questions compiled below represent questions from the CTSA recipient institution community discussed in the CTSA Quality Assurance/Quality Control Group meeting and questions posted in the QA/QC Group Discussion Forum. Responses provided in this document are in accordance with NIH Grants Policy and do not supersede recipient institutional policies.
Last updated: April 2024
KL2/K12/TL1/T32 projects
Q: Should you include the KL2 scholar’s stipend in the NCATS prior approval request for a scholar Human Subjects System (HSS) or Vertebrate Animal Study (VAS) project?
A: The KL2/K12 scholar stipend or salary cannot be included with the KL2/K12 project budget. Please refer to section 2, question 2 of the Human Subjects Research Addendum.
Q: Should a KL2/K12 Scholar be appointed in eRA xTrain before completing the submission of a prior approval request?
A: Yes. Per the terms of award on all KL2/K12 grants, all statements of appointment must be submitted in xTrain before or at the start of each scholar appointment. If a HSS or VAS prior approval is submitted to NCATS before a statement of appointment is submitted in xTrain, NCATS approval will be delayed until the statement of appointment is submitted and accepted. No stipend or other allowance may be paid until the appointment form has been submitted and all grant provided research funds must go to grant supported scholars. It is also helpful if the Authorized Organization Representative (AOR) /Signing Official (SO) notifies the NCATS Grants Management Specialist (GMS) and Program Official (PO) once an appointment is submitted so they may begin the eligibility review.
Q: What happens if a KL2/K12 scholar receives a Research Project Grant (RPG) while appointed on a KL2/K12 grant?
A: The allowability of concurrent support is dependent upon program needs and the eligibility and allowability outlined in NOT-OD-08-065 and The NIH Grants Policy Statement Section 12.3.6.2. Concurrent support must be requested through the recipient institution AOR/SO to the assigned NCATS GMS and PO via a prior approval request. It is important to note that per NOT-OD-08-065 concurrent support can only be considered for an independent research project grant if that application was submitted AFTER the scholar is active on the KL2 or K12 grant.
Q: Can TL1 trainees be pilot project PIs?
A: The TL1 grant is a training mechanism for pre- and postdoctoral fellows preparing for a career in research and is not geared towards individuals engaging in independent research. However, in rare occasions, TL1 trainees (particularly postdoctoral trainees) may conduct independent research supported through the UL1/UM1 Pilot Program Core. The CTSA Program hub must request prior approval for all research conducted by trainees through the Pilot Program that will be independent of the mentor’s research project and ensure proper oversight of the trainee-investigator.
Q: What are the Inclusion Enrollment Report (IER) Requirements for KL2/K12 projects?
A: For the KL2/K12 program, an IER is not required if the scholar is conducting research under a mentor’s project. If the KL2/K12 scholar is conducting an independent clinical study that is not covered under the mentor’s IRB approval and is using KL2/K12 research funds to support the clinical study (including clinical trials), they should alert their Program Officer ahead of time and must follow NCATS guidelines for Human Subject Research prior approvals. Inclusion Enrollment Reports must be updated at the time of the Research Performance Progress Report (RPPR) submission.
Q: What is the IER requirement for KL2/K12 scholars who have their own IRB approval, but the research is also being supported by other grants/funds?
A: If the project is part of a larger NIH supported project that already has IER in place, there is no need to add IER for the KL2/K12 portion.
Q: Can travel funds be used to support TL1/T32 Pre and/or Post doc foreign travel for the purpose of scientific conference attendance?
A: Per the NIH Grants Policy Statement, awarded travel funds may be used to cover the costs of trainee travel, including attendance at scientific meetings, which the organization determines is necessary to the individual’s training. Specific questions regarding foreign travel should be brought to your Authorized Organization Representative (AOR) for guidance and consultation with NCATS if necessary, however it is important to note that any research activity that could rise to a foreign component is not allowed on a training grant.
Q: During Research Performance Progress Report (RPPR) submission, does a trainee progress report need to be completed for a trainee who terminated early and did not complete the appointment period?
A: Yes. This information is required for all trainees and scholars who were appointed to the grant during the reporting period. Please see the NIH RPPR Instruction Guide (PDF - 7.2MB) as well as NCATS Supplemental Instructions for CTSA Program RPPRs.
Q: A KL2/K12 scholar will be going on leave of absence for 3 months. The NIH Grants Policy Statement states that a leave of absence less than 3 months only requires institutional prior approval, and not NIH prior approval. Can there be an extension of the KL2/K12 slot at the end of this fiscal year to cover the 3 months the scholar won’t be working on their project?
A: Scholars are employees of the recipient institution and as such all institutional leave policies will apply. Any changes to the approved scholar slots will require NIH prior approval. Please consult your AOR for any guidance needed regarding a leave of absence and they will advise if the leave of absence requires additional NIH prior approval.
Q: Can a KL2/K12 scholar access their research costs prior to receiving IRB/IACUC approval and subsequently, NCATS Approval, provided those dollars are not spent on human/animal research activities?
A: This depends on the recipient institutional policies regarding the spending of these funds. It is strictly unallowable to spend funds on activities that meet the NIH definition of engagement in human subjects research prior to IRB approval. Activities such as equipment calibration or research planning that does not meet the NIH definition of human subject research activities are generally allowable without IRB approval. It is up to the institution to review and determine if an activity is or is not engaging in human subject research).
Q: How long does it take to review and approve xTRAIN appointments once submitted to NIH?
A: The review process should not take very long assuming there are no eligibility issues with the appointment (scholar and trainee eligibility requirements are included in the applicable Notice of Funding Opportunities). It is imperative that the recipient organization is aware of all awarded and pending grant applications for the trainee/scholar prior to appointment submission to ensure eligibility and avoid delay. Please note that the xTrain system does not notify the grants management specialist (GMS) or the grants management officer when the appointment is submitted in xTrain. Thus, it is recommended to notify GMS and program officer assigned to the grant when the appointments are submitted in xTrain. The notification will ensure there is NIH visibility on the pending appointment/termination and will speed up the review process.
Q: What additional documents are needed for prior-approval packages for scholar projects that marked "Yes" to the question, "Is this study collecting genomic data?"
A: The recipient institution must ensure compliance with the NIH Genomic Data Sharing Policy. Any prior-approval package that indicates the proposed study is collecting genomic data must include all required documentation.
Q: How are the IERs managed for a scholar that leaves the recipient institution? Is there a notation in the IERs indicating they are no longer going to be updated?
A: Recipients may put a comment in the IER comment area if they would like to provide details on enrollment that they believe will be helpful. (For example, to indicate that enrollment stopped early because the scholar left the institution.)
Q: What is the process for transferring projects from the KL2 to K12?
A: The KL2 termination notice and K12 statement of appointment must be submitted to NCATS in xTrain on the same day. This must be done before the project period end date of the KL2 award. The recipient institute AOR should then send an email to GMS and POs of the KL2 and K12 awards when the termination notices and PHS 2271 form have been submitted with the following information:
- Names of the KL2 scholars that were appointed to the K12 in xTrain (VAS and HS).
- Titles of the scholar projects (VAS and HS)
- Associated study IDs (HS only)
- The official NCATS GMS prior approval for the KL2 scholars’ projects if transferring study is category 1 or VAS.
Please note that the NIH GMS does not receive notification when an appointment or termination is submitted so email notification from the recipient institution is appreciated. This notification is not required to come from the AOR, however if there is additional information or documentation required to accept the appointment, AOR concurrence will be needed. For questions, please reach out to Patrick Brown.
Pilots
Q: If a study has several aims with different enrollment criteria per aim, should the PI provide several cumulative enrollment tables?
A: In general, for every planned enrollment report there should only be one cumulative enrollment table.
- Cumulative enrollment represents the cumulative record for all enrollment centers.
- The table should be updated with new records. There are some situations where more than one IER for a study is acceptable: 1) when a study involves both an existing dataset/resource AND recruitment of new participants, or 2) when a study involves multiple, different existing datasets. Please also see the Frequently Asked Questions.
Q: A pilot recipient, whose study received NIH Prior Approval, is leaving the University. The institution would like the study to continue, and they are in the process of identifying a PI to take over the study. How do we process this request through the HSS?
A: This should be discussed with the PO. The recipient institute would need to provide an updated IRB record with the new PI name, along with the HS training record for the PI. There is no need for a new prior approval if this is an ongoing pilot study, that already received NCATS prior approval.
Note: If the PI of the pilot project is also a named key personnel on the parent UL1/UM1 notice of award, this would require a prior approval request on the parent UL1/UM1 grant submitted in accordance with the Change in Key Personnel Prior Approval guidance on the NCATS website. In general, the named key personnel on the parent UL1 notice of award are core directors and personnel that are considered essential to the execution of the award. The recipient should work with their AOR as they are very well versed in this type of prior approval action.
Q: Is reporting pursuant to the Inclusion Across the Lifespan (age at enrollment) policy required for pilots and KL2/K12 projects?
A: The Inclusion Across the Lifespan policy applies to all grant applications submitted for due dates on or after January 25, 2019. Research that was submitted before January 25, 2019, continues to be subject to the Inclusion of Children in Clinical Research Policy.
Q: A recipient is working on RPPR/Progress Report for an exemption 2 study that did not capture age of participants according to the participant-level data template. What is the requirement for reporting?
A: Per the Inclusion Across the Lifespan policy, age data are required for all NIH-Funded clinical research, which includes research that meets the criteria for exemption 2. Investigators are expected to design their studies in a way that allows for the required data collection. As with other inclusion-related variables, participants in research should always have the option to choose not to report their sex, race, ethnicity or age. The participant-level data template allows for age to be left blank for situations where the participant does not provide their age.
Q: If there is a discrepancy between planned and actual enrollment (e.g., enrollment delay) should that be explained in the inclusion enrollment section 2.9 comments section?
A: Yes, the comments should clarify the reason behind the discrepancies in the enrollment numbers.
Q: What are the requirements for reporting the enrollment start and end dates in the Inclusion Enrollment Report in HSS/ASSIST?
A: Enrollment start date is required to be entered for all prospective human subject studies. However, for clinical trials both enrollments start, and end dates are required to be entered. Please follow the instructions in ASSIST. An enrollment start date is not required if the IER is marked as an existing dataset/resource.
Q: Per NIH Policy, “documentation must be submitted that all key personnel have received training in the protection of human subjects”. Who in the recipient institution can submit a signed letter outlining that? Which section of HSS should the attestation go to?
A: NIH policy requires any personnel involved in human subject research to have received training in the protection of human subjects. Recipients may submit actual documentation for all personnel or a letter from an AOR that includes the personnel involved in the project, the title of the training, and the dates of the training. The recipient should upload the documents to section 2.7 in HSS.
Q: Is the Human Subject System (HSS) Section 3.1 an important part of the prior approval document and studies involving human subject research (HSR)?
A: It is an important part of the prior approval document. Please ensure that you follow the template provided in February 2022 QA/QC Meeting Presentation about HSS Section 3.1 and complete the section accordingly. This section should be completed to avoid delay in prior approval process. Even if prior approval is not required, the recipient should ensure that HSS Section 3.1 is addressed, with all necessary sections explained in the February 2022 QA/QC Meeting. This document ensures that the study team is taking necessary steps to ensure participants’ safety and reduce risk.
Q: What is the reporting process for a pilot that needs to be extended to the next budget period?
A: All active HSS and VAS projects supported by NIH research funds and or voluntary committed cost share must be reported in the RPPR. Please reference the NCATS CTSA RPPR Supplemental Instructions for additional clarification. The pilot project activity may cross over budget periods. However, per NIH Grants Policy and the Notice of Award, the institution CANNOT carry over funds from one budget period to another without NIH prior approval. Repeated prior approval requests to transfer funds from one budget period to another for the same/similar program costs will be denied. Please work with your Office of Sponsored Programs to establish your pilot program in a manner that complies with NIH Grants Policy and avoids setting up a need for carryover requests for pilot program funds.
Q: Why should recipients wait for two weeks after notification for category 2 pilots and K12/KL2 projects before starting the project?
A: NCATS POs will confirm that the HSS packet is complete, and the Category 2 designation is appropriate. A Category 2 pilot may start when all materials are submitted via HSS and the documents are being reviewed by NCATS staff BUT if materials are not complete, are not clear, and/or the safety of the Human Subjects appears to be compromised in the study, the study may have to be paused while NCATS is acquiring additional materials. NCATS will communicate this to you through your AOR. Hence, NCATS recommends that the recipient waits two weeks before starting the study to allow NCATS internal review process to complete.
Q: When a recipient submits a new pilot project for prior approval does NIH consider them covered by a Certificate of Confidentiality (CoC)?
A: Yes. See the NIH OER page on CoCs for more information.
Q: Can a recipient submit prior approval request for human subject research and register the clinical trial simultaneously, or do they need NCT # prior to the prior approval submission?
A: Prior approval from NCATS is required before the study starts. Registration to Clinicaltrials.gov needs to be done within 21 days of enrolling the first human subject. When the prior approval request is reviewed by NCATS, the NCT number is not required.
Q: Is a study timeline required for a study under Exemption 4?
A: The study timeline is optional if:
- You selected only Exemption 4 and no other exemptions on the “1.3 Exemption Number” question.
- You answered “No” to any of the questions in the “Clinical Trial Questionnaire” (i.e., your study is not a clinical trial).
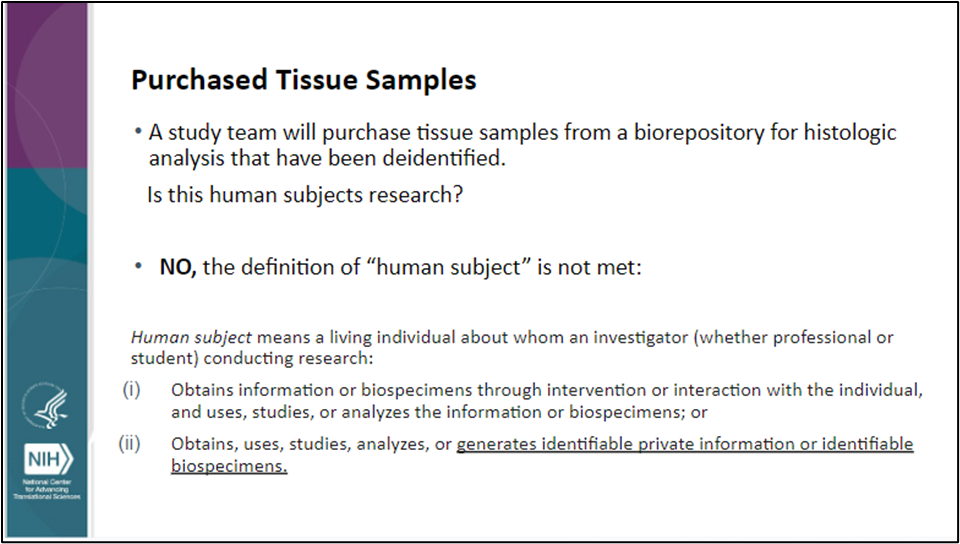
Screenshot of Slide 32 of August 4, 2023, QA/QC Presentation By Dr. Valery Gordon on "Human Subjects Research and Human Research Protections"
Q: In preparation for RPPR submission, are recipients supposed to upload new correspondence for IRB Continuing Review and other new correspondence to original document uploaded to HSS 2.7 “Study Timeline plus the NCATS-specified documents” and replace in HSS?
A: The expectation is that the recipient provides updated inclusion enrollment as well as an updated IRB record (if expired) when updating a study for the RPPR (UL1 (PDF - 726KB) and UM1 (PDF - 680KB)).
Q: A recipient is waiting for prior approval. Is the recipient allowed to start working on the study now using their own personal funds? If so, would the recipient be able to reimburse themselves with NCATS funding once prior approval is granted. The funding will be coming from direct grant dollars.
A: Under no circumstances may NIH-supported non-exempt human subjects research be initiated prior to obtaining IRB approval and providing the final IRB approval date to NIH. NIH will not allow any funds to be used by recipients where a certification and an IRB approval date has not been provided to the funding. NCATS discourage using mixed funds to perform studies in line with the below:
Recipient organizations are not permitted to mix and match sources of funding that have different prior approval requirements. For example, recipient organizations should not partially fund a pilot project with direct funding and voluntary uncommitted cost sharing.
Source of Pilot Funds Prior Approval Required | Prior Approval Required |
Direct Funding | Yes |
Voluntary Committed Cost Sharing Note: UM1 and K12 projects can only be funded with NCATS direct funding. | Yes for UL1 and KL2 |
Voluntary Uncommitted Cost Sharing | No |
Q: Can a UL1/UM1 PI or KL2/K12 PD submit the updates to the IERs instead of their SO?
A: HSS allows for the signing official to delegate authority within the system to the principal investigator for study record submission (see below HSS Roles and Privileges Matrix). There may be some instances in which we need the signing official to either submit or concur submission of a study record in HSS (for instance, the submission of a human subject study record for NCATS prior approval), but we do not require SO submission/concurrence for IER updates. If the PI has delegated authority, they may update IER information and submit via HSS.
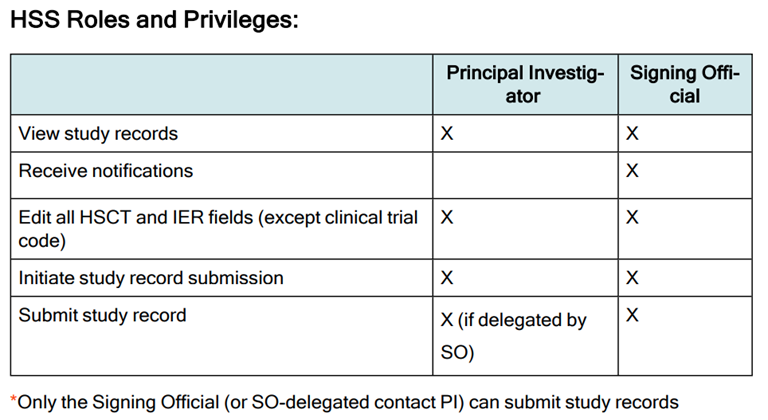
Screenshot of Roles and Privileges Section of the Human Subjects System (HSS) for Institution Staff User Guide
Q: For a category 1 study (greater than minimal risk and/or clinical trial), if we don’t hear back from the NCATS prior approval within 30 days, should the recipient reach out to NCATS?
A: Yes, if recipient does not hear back from the NCATS prior approval within 30 days, they are encouraged to reach out. The recipient can also reach out to the Program Officer with any questions related to the prior approval process.
Q: For category 2 studies, does the AOR need to submit both the submission in HSS and submit the notification/request?
A: All HSS submissions must have AOR concurrence. This can be accomplished in two ways: Concurrence is indicated via the AOR submitting the study request in HSS or if the AOR delegates HSS submission roles within the HSS system the AOR must submit the notification/request clarifying the AOR role to the NCATS Prior Approval Mailbox copying the assigned Program Officer and Grants Specialist.
Q: Do studies using only existing datasets/resources (i.e., no prospective data collection) require participant-level inclusion data?
A: No. Only studies from competing applications submitted for dues dates on or after January 25, 2019, that include prospective enrollment require participant-level data reporting with their RPPRs. Participant-level data is not required for existing datasets/resources. The recipient should only provide aggregate information in the cumulative enrollment table and be sure to mark “Yes” for existing dataset/resource.
Q: How many research project participants are needed for a project to be considered human subject research? If the study includes only a handful of participants, is it still considered a human subject research?
A: It starts at first person. This is because it is about protecting individual subjects from research risk, one of the risks might have to do with identification that subjects have a rare disease, and they don't want everybody to know. Hence, if the recipient knows from the start that they're going to be looking at a particular disease where there are only a very few cases nationally, or internationally, that should automatically be considered human subjects. It is part of the common rule. The common rule does not specify numbers.
Foreign Component
Q: Are foreign components allowed on a pilot project and what steps are needed to approve a foreign component in a pilot project?
A: Please refer to NCATS instructions on submission of prior approval for a foreign component and discuss with the PO.
Q: Is prior approval required for using existing data previously collected outside of the U.S. not using NCATS funding?
A: It’s dependent on where the dataset is located. All the information should be provided to NCATS and it is recommended to discuss with the PO.
Q: Is prior approval required to purchase special purpose equipment needed for research from a foreign vendor under a UL1 pilot project?
A: You must first work with your AOR/SO to determine if the equipment is defined as special purpose and the prior approvals required to move forward (equipment purchase, foreign component). The HSS prior approval submission should include the procurement with a strong justification which will be reviewed within the context of the study protocol.
Vertebrate Animal Studies (VAS)
Q: If a recipient has a vertebrate animal study (VAS), where the animal breeding occurred in another institution, while the research is taking place at the recipient institution, does the NIH prior approval package need to contain IACUC approval from both institutions or just the recipient institution who is euthanizing the animal and doing the in-vitro study?
A: If the breeding is described in VAS section of the application and/or is part of the budget, then IACUC approval from the breeding institution is required. IACUC approval is required for each animal activity described in the grant application. If the breeding is not described in the pilot application, IACUC approval for the breeding protocol does not need to be submitted.
Clinical Trials
Q: If a study record on the ClinicalTrials.gov site is updated, will section 6 of HSS be automatically updated? Additionally, will updates in HSS be automatically populated in ClinicalTrials.gov?
A: : The system does not automatically update, but the system does allow it. The award recipient needs to manually click the populate button to refresh data from clinicaltrials.gov. However, there are things that are checked against the clinicaltrials.gov live. Hence if the HSS information is no longer current, it will lead to warnings and/or errors. Additionally, recipients can export from HSS to clinicaltrials.gov. The export feature will send updates from HSS to clinicaltrials.gov. Again, it is a manual process and is not automatically initiated.
Q: Does a minimal risk study that meets the NIH definition of clinical trial need to be entered into clinicaltrials.gov?
A: Yes, all clinical trials need to be entered into clinicaltrials.gov. A minimal risk study that meets the NIH definition of clinical trial is expected to be registered and entered into the clinicaltrials.gov website.
Q: If the institutional IRB determines an NIH-Defined Clinical Trial to be a minimal risk study, is it submitted for prior approval under category 1 or 2?
A: The recipient should submit the study for NCATS prior approval under category 1. Please refer to the NCATS website.
Q: A recipient has submitted a clinical trial prior-approval request. Is it allowable to begin recruitment without completing study procedures until NIH approval?
A: If the recipient did not receive a formal approval of the prior-approval submission, no interaction with study participants is allowed. Only administrative activities are allowed. The recipient should reach out to the PO to discuss. In general, it should not take more than 30 days for a prior approval to be officially approved by NCATS.
Human Subjects System (HSS) Study Close Out
Q: How can I inform my PO that my study is completed?
A: There is no NIH-wide “close-out” process for study records. However, be aware of any NoFo-specific requirements. NIH expects recipients to:
- Clearly describe study information on PHS Human Subjects and Clinical Trials Information Form
- Keep study status and key study dates up-to-date and accurate.
- Describe study status in progress report, as appropriate.
- It is required to upload clinical trial result information to the Clinical Trials website in compliance with NIH Grants Policy Statement, Section 4.1.3.1. within 1 year of completing clinical trial enrollment.
For more guidance, please refer to the October 2022 QA/QC Meeting on “Documenting Study Activities in the Human Subjects System”, presented by Dawn Corbett, M.P.H., NIH Inclusion Policy Officer from the Division of Human Subjects Research, Office of Extramural Research. View the presentation slides and video.
Q: What are the reporting requirements for pilots that received NCATS funding and continue the study beyond the NCATS funded period, with non-federal funding?
A: Once pilots complete their NCATS funded period, no reporting is required in the RPPR or HSS system. Once a KL2/K12 scholar terminates their appointment, no further reporting is needed in the RPPR or HSS.
All clinical trials must ensure they are compliant with NIH clinical trial reporting requirements regardless of the time period of award.
Q: A UL1 award has ended, and the recipient is now on a UM1 award. If the recipient has a pilot study funded by non-federal money that continued past the UL1 award end date, do the recipient still have to submit a progress report and update enrollment reports in ASSIST?
A: The recipient should note that the UM1 is a different mechanism than the UL1. It is up to the UL1 PIs to complete all reporting requirements during the UL1. The UL1 funding mechanism allowed for pilot studies to be cost shared. The UM1 does not. If a study was supported with cost share under the UL1, it should be closed out and final reports submitted under the UL1. If there are pilots under the pilot project that the hub team wants to continue funding with federal funds (meaning to “transfer” to the UM1 from the UL1), they need to submit in the HSS system of the UM1 and request a new prior approval.
Administrative Supplement
Q: CTSA administrative supplements that involve human subject research. NCATS requires all the human subject documents to be entered into the HSS prior to submission of the administrative supplement, please confirm.
A: The recipient should submit an as complete as possible HSS record when submitting an administrative supplement if it includes human subject research component. Otherwise, the supplement will not have complete documents for the administrative review by NIH.
QA/QC Administration
Data Management and Sharing Plan
Q: Do Pilot Projects submitted to and funded by CTSA's after January 25, 2023, have to include Data Management and Sharing Plan?
A: No, individual Pilot Projects cannot submit a Data Management and Sharing Plan specific to the Pilot Project. All Pilot Projects must comply with the parent award (e.g., UM1) approved Data Management and Sharing Plan. Any changes to the parent award approved Data Management and Sharing Plan requires NIH prior approval.


